51 Toxic Metals and the Environment
Toxic Metals and Their Impact on The Environment
Toxic metals occur naturally in the Earth’s crust and provide necessary minerals to their environment. However, anthropogenic processes disrupt natural feedback loops and concentrate these toxic metals in dangerous amounts. Of the 20 to 30 metals considered toxic, five stand out due to their prevalence and harmful effects, especially in humans: lead, mercury, arsenic, cadmium, and chromium. This chapter explores the primary sources of these toxic metals and their environmental impacts across the atmosphere, hydrosphere, and lithosphere, offering recommendations for minimizing their release.
The source
While natural events like volcanic eruptions can release toxic metals into the environment, human activities are by far the most significant and more damaging source. Mining and metal refining, burning fossil fuels, improper waste disposal, and agricultural runoff are the most common sources of toxic metals.
Mining exposes sulfide minerals, leading to Acid Mine Drainage (AMD). The now-exposed minerals react with water and oxygen, forming acidic solutions that leach toxic metals into waterways. AMD will be discussed in more detail in the hydrosphere section of this chapter.
Smelting, a part of metal refining, releases toxic metals like mercury, arsenic, and cadmium in the process of heating ores. This process also produces slag, a byproduct that holds extremely prominent levels of lead, arsenic, and cadmium. If treated incorrectly, the slag can poison soil, air, and water.
The burning of fossil fuels, especially coal, is particularly harmful. According to the U.S. Environmental Protection Agency (EPA), coal burning accounts for roughly 21% of global anthropogenic mercury emissions. The Union of Concerned Scientists reports that in 2012 alone, the U.S. coal power fleet emitted 41.2 tons of lead, 9,332 pounds of cadmium, and 77,108 pounds of arsenic. Coal-fired power plants are therefore the greatest source of toxic metals pollution globally.
Improper waste disposal causes leaching of toxic metals into the ground or surface water. This is especially prevalent in developing countries where regulations are not as strict. Brazil, for example, has a large electronic waste disposal issue. E-waste goes untreated due to an inadequate waste management system. However, this is not only a third-world problem; developed countries like the United Kingdom struggle with e-waste, often exporting it to poorer countries.
Coal ash is another type of waste that is often improperly disposed of. When handled wrong, it can leach into the groundwater. The Associated Press reports that United States coal ash ponds often lack proper lining, leading to groundwater contamination, such as a devastating 2008 spill in Tennessee. In that instance, a retaining wall at a power plant in the town of Harriman gave way, releasing 545 million gallons of wet coal ash. One official told reporters that the nasty black ash flowed into “the water supply for Chattanooga and millions of people living downstream in Alabama, Tennessee, and Kentucky.” (AP) This case is striking evidence that coal-fired power plants are hazardous and desperately need regulating and retrofitting.
Another more recent case of a mismanaged coal ash pond was recorded in 2022, “A pile of coal waste so big it could fill the Dallas Cowboys’ football stadium twice over threatens groundwater with heavy metal pollution.” (AP)
The agricultural sector in the United States is another large contributor to the toxic metal problem. Runoff from livestock waste, wastewater, fertilizers, and pesticides all contribute to the levels of toxic metals in freshwater, groundwater, soil, and sediments.
Chromium behaves somewhat differently than the other four toxic metals discussed in this chapter, which earns it a section here. While naturally present in coal, it only transforms into its most dangerous form (Hexavalent Chromium) under high temperature combustion, such as in welding. This carcinogenic form is heavily used in industries such as chrome plating and steel production. Improper disposal of industrial waste allows hexavalent chromium to contaminate nearby water sources, a problem compounded by the rising tide of electronic waste.
Understanding the source of toxic metals is important because removing them from the environment is difficult. It is far easier to stop them at the source than to try to remove them from the environment after the fact. This is due to the persistent nature of toxic metals, making them so dangerous. The EPA sets the safe threshold for cadmium at five parts per billion. This is quite small, and you can see how consistent small exposure to cadmium could exceed this threshold. This is not just cadmium. “Exposure to mercury, even in small amounts, may cause serious health problems.” (WHO) This threshold was made for human exposure, however, no amount of mercury or other toxic metals is safe for the environment. Years and years of steady pollution of toxic metals cause devastating effects on our environment. “Heavy metal pollution will increase the risk of extinction for vulnerable species as a result of increased heavy metal pollution.” (Sharma, Kant, and Sharma, 2025). Heavy metals like the ones described in that article are often used interchangeably with toxic metals, but they are not quite the same. Heavy metals are metals that have a mass five times greater than water. All toxic metals are considered heavy metals, but not all heavy metals are toxic, such as gold, which is considered a heavy metal but not a toxic metal. Still, the point remains, toxic metals are hazardous to all environments in any amount.
Arsenic, lead, mercury, and cadmium all originate disproportionately from coal-fired power plants. Either in the emissions or in the disposal. Chromium originates from improperly disposed of industrial waste. Together, these five toxic metals pose a significant threat to living beings.
The atmosphere
The atmosphere is composed of five main layers; this chapter focuses on the troposphere, the only layer that houses life. Toxic metals do not degrade or alter the atmosphere in any way, but they do still pose a threat. When coal containing toxic metals is burned, pollutants such as mercury, lead, and arsenic are released into the atmosphere. These pollutants return to Earth through rain or as dry ash, contaminating ecosystems anywhere they land. Mercury is particularly concerning because it is emitted as a vapor, enabling it to travel great distances before depositing in soil or water.
Arsenic, lead, and cadmium are typically emitted as fine particulates that can still drift hundreds of kilometers but often settle nearby. These airborne toxic metals degrade air quality, pose respiratory and cardiovascular risks, and contribute to regional and global pollution. Air patterns in the troposphere can circulate mercury great distances. Meaning the emissions from one country will harm neighboring regions. Mercury’s capacity to spread globally makes international cooperation vital.
One global cooperation effort that changed the toxic metals’ effect on the atmosphere was removing leaded gasoline. The UN says that “Official end of use of leaded petrol will prevent more than 1.2 million premature deaths and save USD 2.45 trillion a year.” (UN) In 1970, all gasoline produced around the world contained lead. The UN Environment Programme (UNEP) classified it as one of the most serious environmental threats to human health. Fifty years later, gas stations in Algeria stopped providing leaded gasoline, ending the use of leaded gasoline globally. The leaded gasoline phase-out is an example that global cooperation to limit airborne pollutants is possible. Another example is regulations limiting the release of sulfur dioxide by coal power plants. Governments required technologies that reduce sulfur dioxide emissions from coal-fired power plants, leading to large reductions in sulfur dioxide in the air, largely solving the crisis of acid rain. These case studies prove that global cooperation is possible.
However, the atmosphere is still plagued by emissions from industrial plants, smelting, and fossil fuel combustion. Despite advancements, more action is needed. While toxic metals do not degrade the atmosphere, they do negatively affect the air you breathe.
The Hydrosphere
The hydrosphere includes all water on Earth: oceans, rivers, lakes, groundwater, and glaciers. Toxic metals reach these waters through atmospheric deposition and industrial discharge, among other pathways.
One study led by the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) measured the levels of mercury, cadmium, and lead in fish, shellfish, and sediment between 1979 and 2020 by monitoring sites of fish, shellfish, and sediments throughout Arctic waters. The results are shown below in two figures.
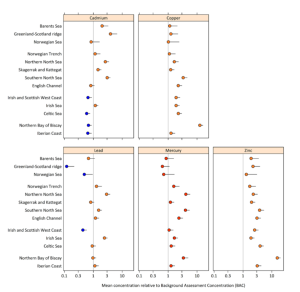
Figure 1: Modeled metal concentration and their 95% confidence limit in fish and shellfish mercury, zinc, cadmium, copper, and lead relative to BAC. Blue dots indicate below background, green dots indicate below environmental criteria, and red dots indicate above environmental criteria. Yellow/orange dots indicate just below or at the environmental criteria. Reproduced from Status and Trends for Heavy Metals (Mercury, Cadmium and Lead) in Fish, Shellfish and Sediment, by OSPAR Commission, 2023, Quality Status Report 2023. https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/heavy-metals-biota-sediment/
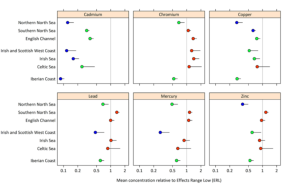
Figure 2: Modeled metal concentration for zinc, copper, lead, mercury, cadmium, and chromium in sediment relative to ERL. Blue dots indicate below background, green dots indicate below environmental criteria, and red dots indicate above environmental criteria. Reproduced from Status and Trends for Heavy Metals (Mercury, Cadmium and Lead) in Fish, Shellfish and Sediment, by OSPAR Commission, 2023, Quality Status Report 2023. https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/heavy-metals-biota-sediment/
Figure 2 shows that the sediment also has levels of mercury above the environmental criteria, along with other pollutants. These figures, taken from the OSPAR, show that despite the regulations and global conventions on mercury, we still see an alarming level in many oceans around the world.
Figure 1 shows that mercury levels in fish and shellfish are above the environmental criteria in every one of the waters tested.
These results are particularly concerning due to mercury’s ability to accumulate in animal tissue. This bioaccumulation causes its concentrations to increase throughout the food chain. Animals at the top of the food chain have higher concentrations than those in lower levels. But it is not just the larger animals that are at risk of mercury poisoning. Microorganisms in the soil or water can convert mercury into methylmercury (MeHg). This organic form of mercury is more bioavailable than other forms and is quickly absorbed and slowly excreted. In aquatic animals, this absorption happens through feeding. (Arcagni et al., 2018) MeHg is a potent neurotoxic compound, inducing oxidative stress and neuroinflammation. This also adversely affects the genome, reproduction, and various body systems in humans and animals (Arcagni et al., 2018). Therefore, the contamination of aquatic organisms by mercury is of concern both ecologically and in a public health context (Evers et al., 2008).
Minamata Bay, Japan, was one of the more deadly cases of mercury poisoning. “On May 1, 1956, a doctor in Japan reported an epidemic of an unknown disease of the central nervous system, making the official discovery of Minamata disease.” (Health and Environment Alliance) A nearby factory dumped large amounts of mercury into the bay, where it was transformed by bacteria in the water into methylmercury. This was biomagnified by fish and then eaten by the local population. Fetuses and children were the most at risk and “over two thousand people died, thousands more experienced crippling injuries.” (Health and Environment Alliance)
Mercury is not the only toxic metal polluting the Earth’s waters. Cadmium, while not in large concentrations in the Arctic Ocean, is very concentrated in certain freshwater bodies. A recent study published in March 2025 on sediment contamination in urban areas of the Yellow River, China, concluded that “excessive cadmium levels were a direct result of industrial and agricultural production. Cadmium contamination has rendered significant portions of the river uninhabitable.” (Junzhang Wang, Et. Al) This is not confined to China but is echoed in polluted waterways around the globe.
The lithosphere
The lithosphere consists of Earth’s rock, sediments, and soils. While toxic metals like arsenic, lead, cadmium, and mercury naturally reside underground, they are unearthed through human activity (mining and quarrying), leading to soil contamination.
Two key terms illustrate the environmental toll of mining:
- – Overburden: the layer of soil or rock that must be removed to access ore deposits
- – Tailings: the leftover material after valuable metals have been extracted
Overburden and tailings often contain high concentrations of toxic metals. These wastes are susceptible to weathering, which leaches metals into surrounding soils. Over time, this contamination can render the land infertile and unsuitable for agriculture or habitation. As the world’s population rises, land becomes increasingly sought after, making this issue more concerning.
What exactly is happening to the soil that makes this area inhabitable? Toxic metals kill the microorganisms in the soil, which are responsible for biological processes such as nutrient cycling, organic matter cycling, and carbon sequestration. Without these necessary microorganisms, the soil becomes infertile, and life cannot exist.
Two scientists, Raiesi and Sadeghi, studied the interactive consequences of cadmium and salinity on soil microbes and enzymatic activities.
The combination of cadmium and salinity caused a reduction of microbial respiration and the content of microbial biomass in soil. (Raiesi, F., Sadeghi, Et al. 2019)
Another scientist went further in their study and found that, “The effects of lead on soil are several, such as reducing soil nutrients, microbial diversity, and soil fertility.” ( Alengebawy, et al 2021) “The combined toxic effects of lead and cadmium were obvious in the number of bacteria and actinomycetes, which were notably reduced.” (Anya et al.) “Cd causes inhibition of mineral transportation and negatively affects the plant’s microbial growth. Pb accumulation in plants causes DNA damage, chlorophyll content reduction, and inhibition of seed germination.” (Anya et al.)
The current literature on toxic metals in the soil is well studied and understood. They negatively affect almost every aspect of the soil, leaving it useless. You don’t have to look far to see how devastating these metals are. Tar Creek Superfund Site in Ottawa County, Oklahoma, is a textbook example of a mismanaged mining project that caused historic lead, zinc, and cadmium contamination. Tar Creek is an 11-mile-long contaminated waterway plagued by piles of mine tailings containing toxic lead dust. The area has approximately 30 million tons of mine tailings containing lead dust, with some mounds towering to nearly 200 feet. (EPA)
Due to its proximity to the Tar Creek Superfund site, members of the Quapaw Nation have been historically and disproportionately impacted by Tar Creek’s contaminated waters. “In 1994, test results from the Indian health service showed that 35% of Native American children in the area had concerningly high blood lead levels.” (EPA) “Another area study that examined young school children showed that they had over 43% elevated blood lead levels, 11 times the state average.” (EPA). Quapaw Nation residents were forced to move from their ancestral home in Arkansas to northeastern Oklahoma. They lived there long before lead was discovered, and mining operations began.
The Quapaw people have been forced out of their home twice now by the federal government. This land was once valuable and rich in resources, but now it must be completely leveled; it will not be safe for decades.
Another example of toxic metals affecting soil happened in the Hunan Province, China. Cadmium was found in rice fields, which led to cadmium rice entering the food chain in 2013. (Kaiman, 2014) Hunan is China’s largest rice-producing province and a hub for metal mining. Cadmium from the metal processing and mining leaches into the rice fields and is taken up by the grains. This disaster in 2013 prompted a national soil report published by China’s Ministry of Land, which said that arable land was continuing to be lost to pollution, urbanization, and industrialization. The vice minister, Wang Shiyuan, admitted that a further 8.2 million acres of arable land was moderately polluted but still in use. (He, 2014) It seems that the lack of enforcement allows untreated wastewater to be used to irrigate farmland. This loss of arable land is a huge issue for China, especially as its population continues to rapidly grow.
Toxic metal pollution is often slow, yet still devastating. Mining activities introduce dangerous levels of cadmium, lead, and arsenic into valuable soil. Real-world examples such as the Tar Creek Superfund Site in Oklahoma and Cadmium rice fields in Hunan, China, reveal how toxic metal exposure doesn’t just turn valuable land useless but also dangerous. This sustainability crisis, if left unchecked, will become irreversible.
Limiting toxic metals in the environment
Throughout this chapter, coal-fired power plants have been highlighted as the largest source of toxic metals. Coal is a cheap form of energy, but it lacks sustainability. The best choice for the environment would be to greatly limit or stop using coal-fired power plants, but that is unrealistic, as many depend on the electricity produced by these plants. However, there exist technologies that reduce the pollution from coal-fired power plants dramatically.
The most common control technologies in use for reduction of air toxics from coal-fired power plants are electrostatic Precipitators (ESP), Wet or dry flue gas desulfurization (Scrubbers), and baghouses.
ESP uses particles charged with electricity and collected on oppositely charged plates; particles are collected for disposal/further treatment. A majority of coal-fired power plants use ESP, but it can still be implemented in other countries. Another common control technology is flue gas desulfurization or scrubbers. This involves spraying a liquid mixed with limestone into the emissions. The purpose of these scrubbers is to turn the emissions into soil salts, which can be removed more efficiently. This technology is used more in plants that use coal with higher amounts of sulfur.
The simplest control method is a Baghouse. Fabric filters trap the nasty stuff, which is collected into hoppers to be adequately disposed of. The last control technology discussed in this chapter is Activated Carbon Injection (ACI). Activated Carbon Injection utilizes activated carbon to control mercury, arsenic, chromium, and other organic carbon-based gas compounds.
Forcing these plants to use ACI and a Baghouse would significantly improve the capture of fine particles and metal-laden ash. The United States, the EU, and certain regions of China have implemented these controls, but many other countries have not. As outlined previously, limiting toxic metals in the environment is a global effort, meaning countries that are more developed must help lagging countries retrofit their power plants. Helping developing nations install ACI, ESP/Baghouse would significantly lower mercury emissions globally. Developing countries usually depend more on dirtier forms of electricity as it is cheaper. Giving these countries the necessary funds for more sustainable energy options makes energy choices simple. As outlined earlier in the chapter, toxic metals do not abide by national borders, making global cooperation crucial. Making sustainable energy options more affordable is just one example of global cooperation to limit the spread of toxic metals.
What would replace coal? This is a difficult question as power demand and energy storage can vary around the world. For example, northern countries need far more energy to heat their homes than countries closer to the equator. Even countries farther north can switch to mainly renewable energy sources. Finland relies heavily on wind and nuclear power and is attempting to switch from district heating, which uses fossil fuels, to heat pumps. This would mean a large increase in Finland’s wind and nuclear capacity, but it is doable. The takeaway here is that a diverse energy district centered around renewable energy sources like solar, wind, hydrogen, nuclear, and hydroelectric can replace coal. This shift away from coal is necessary if we want to ease the burden of toxic metals.
The sources section of this chapter outlined the main sources of toxic metals, how they find their way into our air, water, and soil. Many regulations are in place to limit these exposures, five of the most important are listed here.
- – National Environmental Policy Act
- – Clean Air Act
- – Clean Water Act
- – Comprehensive Emergency Response and Cleanup Act
- – Inflation Reduction Act
These laws are paramount in fighting toxic metals. The EPA uses these laws to hold polluters accountable and to discourage future pollution. The National Environmental Policy Act “Requires agencies to disclose the environmental effects of their actions.” (Legal Planet). This has held many polluters accountable and is one of the older environmental laws. The Clean Air Act provided the basis for the EPA’s regulation of greenhouse gases. (Legal Planet). This is the most important environmental law, as air pollution exposes billions to harmful effects. The Clean Water Act “gives the EPA authority to set limits for water pollutants, help fund wastewater infrastructure, and support research and development.” (not sure if this is a quote). This has been particularly limited in recent years, and lawyers have argued about what bodies of water should be protected and not. The Comprehensive Emergency Response and Cleanup Act provides cleanup of hazardous waste sites. (Legal Plant). This is also known as the Superfund law, This law was especially helpful in cleaning up the Tar Creek Superfund site, which was mentioned earlier in the chapter. Lastly, the Inflation Reduction Act provided about $370 billion for clean energy and climate-related programs. This controversial act funded many programs that will reduce the toxic metals’ burden on the environment.
Advocating for lawmakers to strengthen these five environmental laws will significantly help fight off toxic metals. It can be difficult to remove these metals from the environment so strong regulations are crucial in saving the environment. These five laws are weaker than ever, and therefore, the world’s air, water, and soil are vulnerable to toxic metal poisoning.
