8 What is air pollution- A Breif Background

What Is Air Pollution
Attributed as a cause of death for 8.08 people in 2021, according to OurWorldInData.org, air pollution is the second largest risk factor in the world, right under high blood pressure. The EPA defines air pollution as one or more chemicals or substances in high enough concentrations in the air to harm humans, other animals, vegetation, or materials. (EPA) The World Health Organization defines air pollution as contamination of the indoor or outdoor environment by any chemical, physical, or biological agent that modifies the natural characteristics of the atmosphere. (WHO) Air pollution is an issue that affects every living person, and it is a shared responsibility to maintain clear, breathable, safe air. (A.B)1,11
What Causes Air Pollution
The combustion of fossil fuels, industrial facilities, and forest fires are common sources of air pollution. When considering what causes air pollution, regulating bodies often focus on what they term, Criteria Pollutants when deciding reduction measures. Criteria pollutants are the major air pollutants that regulators look to manage as they are the most likely to cause harm to humans or the environment. The following section will go over the major criteria pollutants. (A.B)1
Types of Air Pollutants
PM (2.5 and 10)
In the last couple of years, fires have raged across the United States. The great Canadian wildfires and then the California wildfires. A haze filled the sky and obstructed vision, the primary cause of this haze, particulates given off in the form of wildfire smoke. Particulate matter consists of
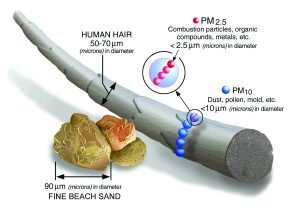
Microscopic solids/liquid droplets that are so small that when inhaled, they can travel deep into the lungs and cause serious health problems. Particulate matter (PM) can be broken down into two types, fine and coarse.
The difference between the two types is size and effect on the body. Coarse PM (PM 10) consists of particles between 10-2.5 µm in diameter. Sources of these particles consist of pollen, sea spray, and windblown dust. The more dangerous, fine PM (PM 2.5) consists of particles less than 2.5 µm in diameter. PM 2.5 is generated through the combustion of fuels in power plants, manufacturing, or vehicles. A secondary source of PM 2.5 is through chemical reactions between gases. PM 2.5 is more dangerous because it is so small that it can pass through the lungs into the bloodstream and cause problems throughout the body. In humans, it causes strokes, heart disease, and repository attacks. (A.B.)1,2
Carbon Monoxide (CO)
The silent killer of many households, Carbon Monoxide (CO) is a colorless, odorless gas that takes lives without any warning. It is produced by the incomplete combustion of several fuels such as wood, coal, and natural gas. Many household products (furnaces, generators, and charcoal grills) give off carbon monoxide without even realizing it. The primary contributor to carbon monoxide in the atmosphere is motor vehicles. With the number of motor vehicles in the world expected to

be around 1.47 billion vehicles, carbon monoxide is a major contributor to climate change in the world. In humans, carbon monoxide is deadly because it diffuses across the lung tissue into the bloodstream, prohibiting oxygen from binding to cells. This leads to a lack of oxygen, causing asphyxiation, which causes tissue damage and cell death. Common symptoms of carbon monoxide poisoning are described as “flu-like.” Symptoms include headaches, dizziness, weakness, upset stomach, vomiting, chest pain, confusion, and then finally, the worst case, death. The deadliest combination includes sleeping intoxicated while also breathing in carbon monoxide. Those who sleep intoxicated may not even notice signs of carbon monoxide poisoning and die in their sleep. Carbon monoxide detectors can be used in households to help reduce the risk of exposure. (A.B.)1,3,4
Nitrogen Dioxide (NO2)
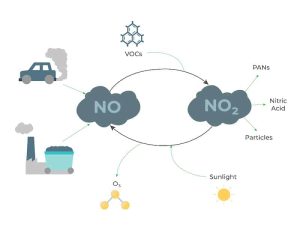
A red-brown gas that is soluble in water, a strong oxidant, an inorganic compound, a highly reactive and poisonous gas. Nitrogen Dioxide is a pungent and extremely volatile, dangerous air pollutant. Nitrogen dioxide is strongly associated with fossil fuel combustion. Nitrogen dioxide is formed when combustion temperatures are high enough to cause nitrogen (N2) in the air to react with oxygen(O2). Major polluters such as coal/gas-fired power plants and gasoline engines. Nitrous oxides are also a major source of Ozone in the atmosphere. When NO2 molecules meet VOCs, they form O3. Nitrous dioxide is also the cause of acid rain. The Nitrous dioxide mixes with the precipitation and decreases the pH of the water as it falls back down. Exposure to nitrogen dioxide can irritate airways and aggravate respiratory conditions. It has also been closely linked to asthma and other respiratory conditions. (A.B.)2,5
Sulfur Dioxide (SO2)
Another gas that is readily soluble in water, sulfur oxides, the greatest of our concern is SO2, or Sulfur Dioxide. Not much different than NO2, SO2 is given off from the combustion of fossil fuels when sulfur is given off by fuels and binds with oxygen in the atmosphere. SO2 is used as the indicator for the larger group of gaseous sulfur. SO2 is used as it is found in the highest concentrations as well as control measures targeting SO2 have been found to reduce all sulfur oxide concentrations.
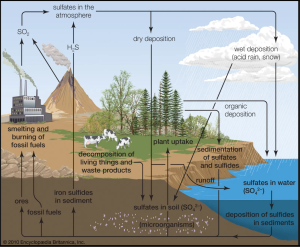
Exposure to SO2 is associated with asthma hospital admissions and emergency room visits. Other sources of SO2 pollution include processes such as extracting metal from ore, volcanoes, and machines/vehicles that burn fuel with a high sulfur content. The health consequences of short-term exposure include respiratory irritation that adversely affects those with conditions and especially children. SO2 is also a contributing factor in the formation of haze, acid rain, as well as damaging plants by decreasing foliage and growth. (A.B.)1,7
Lead
One of the more famous pollutants in recent years, from stories of entire towns being poisoned to stories of children developing neurodegenerative diseases after eating it off their walls. Lead is a dangerous toxin that can be found in the air, water, and as solids in many products. Lead is a naturally occurring metal found in the crust of the earth, but many of its dangers are from anthropogenic uses such as gasoline, paint, water pipes, ammunition, and ceramics, to name a few. Lead can also be sourced from industrial and contaminated sites, such as former lead smelters. Dangerously, there is no safe level of lead in the body, and exposures as low as 3.5 µg/dL may be associated with decreased intelligence.

In the body, blood carries only a small fraction of the total lead body burden, distributing it throughout the body, making it available to other tissues. Most of the lead that is absorbed ends up in the Mineralizing tissues (bones and teeth). This is because in the body, lead mimics calcium. Lead can affect almost every organ and system in your body. Children who are six years old and younger are most susceptible to the effects of lead because they are more likely to put things into their mouths and have less developed systems that are more susceptible to the effects of lead. In children, lead has been found to cause health effects such as behavior and learning problems, lower IQ and hyperactivity, slowed growth, hearing Problems, and anemia. Pregnant women are also at risk, as lead will build up in their bodies over time and then be passed on to the fetus. Effects on the fetus include, baby being born too early or too small, damage to the baby’s brain/kidney/ nervous system, an increased likelihood of learning or behavioral problems, and an increased risk for miscarriage. Even in healthy adults not at risk, lead can cause cardiovascular effects, as well as reproductive damage and kidney damage. Lead is a dangerous toxin in the environment, and while it is being eliminated where it can, it is still important that houses be checked for lead paint as well as lead pipes, and individuals be informed so they can better protect themselves from exposure. (A.B.)16,17
Radon (Rn)
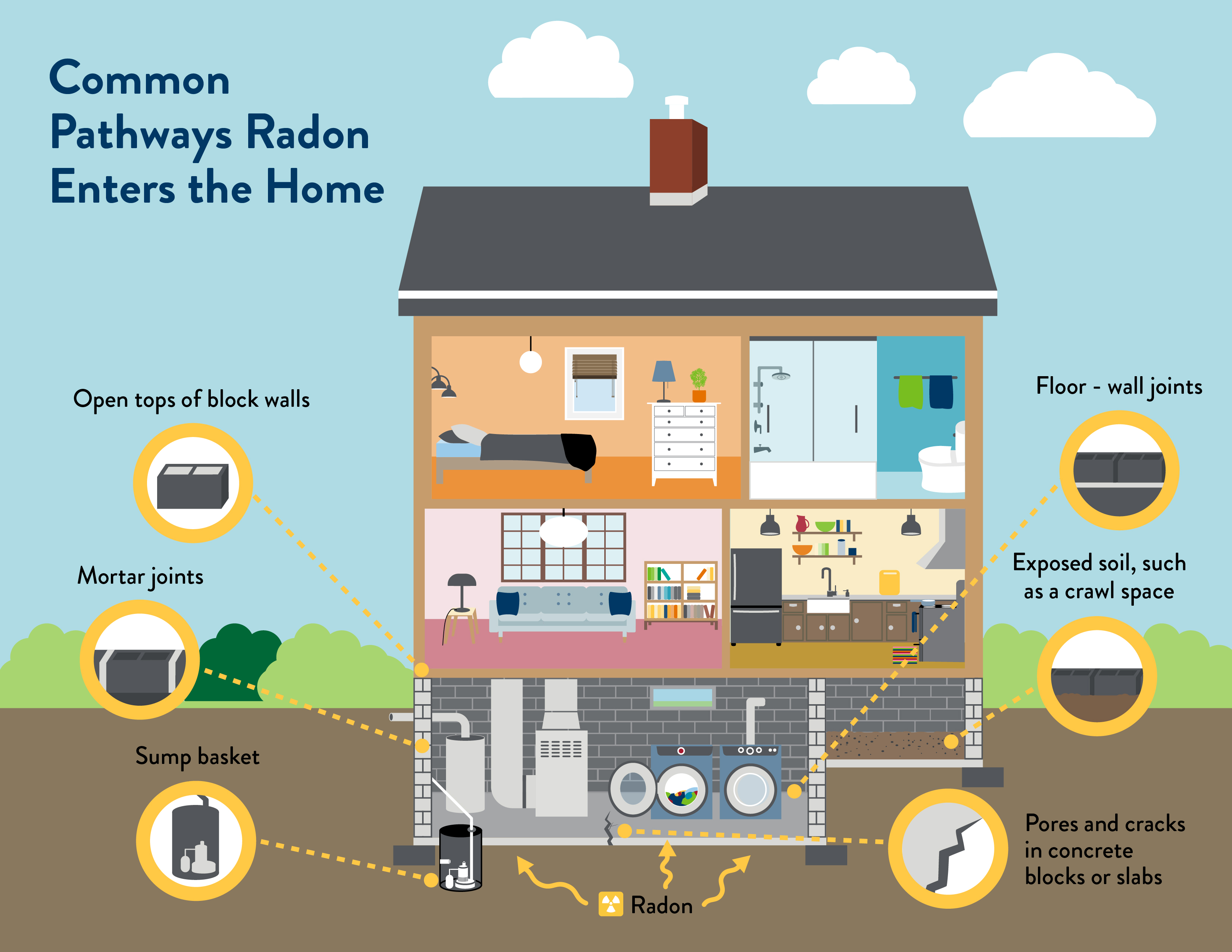
Radon is the number one cause of lung cancer among non-smokers and is responsible for about 21,000 lung cancer deaths every year. Radon is a colorless, odorless, radioactive gas that forms in the ground when radioactive metals break down in rocks, soil, and groundwater. Radon gas breaks down into tiny radioactive particles that can be breathed in and lodged in the lining of the lungs, where they can give off radiation. This radiation damages lung cells and eventually leads to lung cancer. The main exposure pathway of radon includes breathing radon in the air, radon that slips through cracks in buildings, usually at ground levels or sublevels. Radon can also be found sometimes in well water as well as certain building materials. Any home can have a radon problem, and it is important to have homes regularly tested for radon. Radon testing kits can be found online. Radon is especially dangerous for those who are smokers, this is likely due to the combined effects of carcinogens. The EPA and the WHO both have radon action plans and are working to actively inform and increase testing for radon. (A.B.)18,19
Ozone
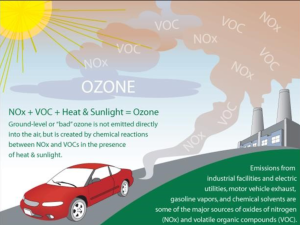
The double-edged sword of air quality, ozone(O3), has two distinctions that mean the difference between helpful and hurtful. The distinctions include stratospheric and tropospheric. In the stratosphere, ozone plays an important role in blocking the sun’s harmful UV radiation. On the other hand, ozone present in the troposphere (the air we breathe) when inhaled reacts chemically with the respiratory system, leading to several adverse health effects such as coughing, throat irritation, pain, burning, or discomfort in the chest when breathing, chest tightness, wheezing, and shortness of breath. A common misconception surrounding tropospheric ozone is that it is only high in urbanized areas. While high ambient ozone concentrations can and do occur anywhere, ozone can travel hundreds of miles from where it originated. Ozone is tricky to control as it is not directly emitted; in the stratosphere, it forms from the interaction between naturally occurring O2 and UV radiation. In the troposphere, ozone is formed from photochemical reactions between volatile organic compounds (VOC) and nitrogen oxides (NOx). Because it is not directly emitted, it makes ozone especially tricky to control without regulation of the reactants that make ozone. Luckily, mitigation efforts for VOCs and NOx will see a reduction in ozone formation that leads to a killing of two birds with one stone situation. The best way to avoid the harmful effects of ozone is to check the air quality rating before going outside and take protective measures when going outside during poor quality periods. (A.B.)19
Volatile Organic Compounds (VOCs)
Volatile Organic Compounds (VOCs) are compounds that have high vapor pressure and low water solubility. They are not a single contaminant or pollutant but rather are given off by a range of products that exceeds the thousands. Typically, they are found in the form of industrial solvents, liquid fuels, and byproducts from chlorination in water treatment. VOCs are also a common groundwater contaminant. They are also found in high concentrations in households, about 2-5 times higher than outdoors. This number also does not matter if the home is in a large city or a small rural area.  This is because many household products, such as paints, glues, cleaning supplies, pesticides, and many other products, give off VOCs during use and some during storage. While breathing in low levels of VOCs for a long period can increase the risk for health problems, there is a greater risk when the levels are elevated. Breathing in high levels of VOCs can lead to eye, nose, and throat irritation, headaches, nausea/vomiting, Dizziness, and worsening of asthma symptoms from acute exposure. Chronic exposure to VOCs can lead to liver and kidney damage, nervous system damage, and cancer. There are various ways to reduce exposure to VOCs, including storing them away from the home in a garage or separate structure, purchasing low-VOC options, only buying products when they are needed, and increasing fresh air in the house. (A.B.)20,21
This is because many household products, such as paints, glues, cleaning supplies, pesticides, and many other products, give off VOCs during use and some during storage. While breathing in low levels of VOCs for a long period can increase the risk for health problems, there is a greater risk when the levels are elevated. Breathing in high levels of VOCs can lead to eye, nose, and throat irritation, headaches, nausea/vomiting, Dizziness, and worsening of asthma symptoms from acute exposure. Chronic exposure to VOCs can lead to liver and kidney damage, nervous system damage, and cancer. There are various ways to reduce exposure to VOCs, including storing them away from the home in a garage or separate structure, purchasing low-VOC options, only buying products when they are needed, and increasing fresh air in the house. (A.B.)20,21
Refenrcens
-
“Air Pollution.” World Health Organization, World Health Organization, 2024, www.who.int/health-topics/air-pollution#tab=tab_11
-
“Carbon Monoxide Poisoning Basics.” Carbon Monoxide Poisoning, 16 May 2024, www.cdc.gov/carbon-monoxide/about/index.html.4
-
“Does Radon Cause Cancer?” Www.cancer.org, American Cancer Society, 1 Nov. 2022, www.cancer.org/cancer/risk-prevention/radiation-exposure/radon.html.19
-
“Lead Poisoning and Health.” Who.int, World Health Organization: WHO, 27 Sept. 2024, www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health.17
-
“Learn about Lead.” US EPA, United States Environmental Protection Agency, 20 Aug. 2018, www.epa.gov/lead/learn-about-lead.16
-
“Nitrogen Dioxide | Chemical Compound | Britannica.” Www.britannica.com, www.britannica.com/science/nitrogen-dioxide. Accessed 7 Feb. 2025.5
-
“Particulate Matter (PM) Basics.” United States Environmental Protection Agency, 11 July 2023, www.epa.gov/pm-pollution/particulate-matter-pm-basics.2
-
“Radon | US EPA.” US EPA, 22 Apr. 2019, www.epa.gov/radon.18
-
Ritchie, Hannah, and Max Roser. “Air Pollution.” Our World in Data, Global Change Data Lab, Feb. 2024, ourworldindata.org/air-pollution.11
-
Stumpf, Rob. “Here’s about How Many Cars Are There in the World in 2023.” The Drive, 17 Oct. 2023, www.thedrive.com/guides-and-gear/how-many-cars-are-there-in-the-world.3
-
“Sulfur Dioxide Basics | US EPA.” US EPA, 16 Feb. 2023, www.epa.gov/so2-pollution/sulfur-dioxide-basics.7
-
“What Are Volatile Organic Compounds (VOCs)?” US EPA, 19 Feb. 2019, www.epa.gov/indoor-air-quality-iaq/what-are-volatile-organic-compounds-vocs.21
-
“What Is Ozone?” US EPA, 21 Mar. 2016, www.epa.gov/ozone-pollution-and-your-patients-health/what-ozone.19
-
“Volatile Organic Compounds (VOCs) in Your Home – EH: Minnesota Department of Health.” State.mn.us, 2019, www.health.state.mn.us/communities/environment/air/toxins/voc.htm.20
