7 Energy and Matter
Big Ideas and Big Questions
Big Ideas
- Energy can move throughout a system.
- The properties of matter can effect how systems interact.
Big Questions
- What are the different ways energy can impact matter?
Heat Transfer
The term “heat” as used in everyday language refers to both thermal energy (the motion of atoms or molecules within a substance) and the transfer of that energy from one object to another. In science heat refers specifically to the energy transferred through the temperature difference between two objects. Heat transfer occurs in three different ways: conduction, convection, and radiation (figure 13). Each of these transfers can impact the kinetic energy (moving energy) of the molecules and atoms within the objects, or systems, that are interacting. When the systems are in contact with each other, the molecules or elements from a high energy (hot) source transfer energy to a lower energy source (cool). For example, when placing an ice cube on a hot piece of metal, the atoms in the hot metal transfer their thermal energy (heat) to the ice’s molecules causing them to move faster. As the molecules in the ice cube move quicker, they increase the internal thermal energy which may causes a phase change with the solid ice turning to liquid.
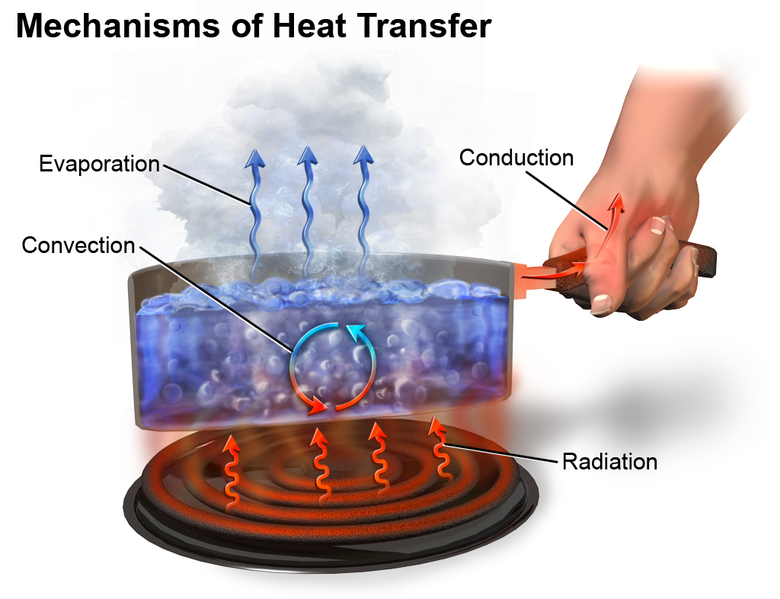
Figure 13: Heat Transfer
(Blaus, 2017)
Convection
is the transfer of thermal energy through the movement of a group of particles in a liquid or gas.
Conduction
is the transfer of thermal energy through direct contact.
Radiation
is the transfer of thermal energy through thermal emission.
Conduction, Convection, and Radiation Video
Click here to watch a video about Conduction, Convection, and Radiation
Teacher Time Out: Heat Transfer
How does this connect to the NGSS Progression
DCI: PS3.A Definition of Energy: Students begin to explore this concept in grade band 3-5 where they focus on the movement of an object from place to place and the varying ways energy can be transferred (light, sound, waves, etc.). Students begin to dig deeper into kinetic and potential energy within states of matter in grade band 6-8 as they explore the idea of temperature as movement of molecules and the energy that is transformed.
DCI: PS3.B Conservation of energy and energy transfer: Grade bands K-2, 3-5, and 6-8 have students build on energy transfer concepts as students explore where energy can come from, how that energy can be felt with the senses, and the varying ways energy can be transferred.
|
|
K-2 |
3-5 |
6-8 |
| PS3.A Definitions of energy | N/A | Moving objects contain energy. The faster the object moves, the more energy it has. Energy can be moved from place to place by moving objects, or through sound, light, or electrical currents. Energy can be converted from one form to another form. | Kinetic energy can be distinguished from the various forms of potential energy. Energy changes to and from each type can be tracked through physical or chemical interactions. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter |
| PS3.B Conservation of energy and energy transfer | Sunlight warms Earth’s surface |
Implementation into the Classroom
Investigation lesson where students work to discover what does, and doesn’t, affect the melting of ice
Engineering lesson where students work to design a way to slow the melting of ice using what they’ve learned about properties of matter
Chemical Reactions and Physical Changes
Matter interacts in everyday life and these interactions can lead to the formation of new products. This process is called a chemical reaction or a chemical change. A chemical reaction is the process of one or more reactants (what’s put in or mixed together) converting into one or more new substances (products) (figure 14). When chemical reactions occur the atoms stays the same, but the energy is transferred to break apart atoms or to join atoms together to form new arrangements of atoms and therefore new types of matter.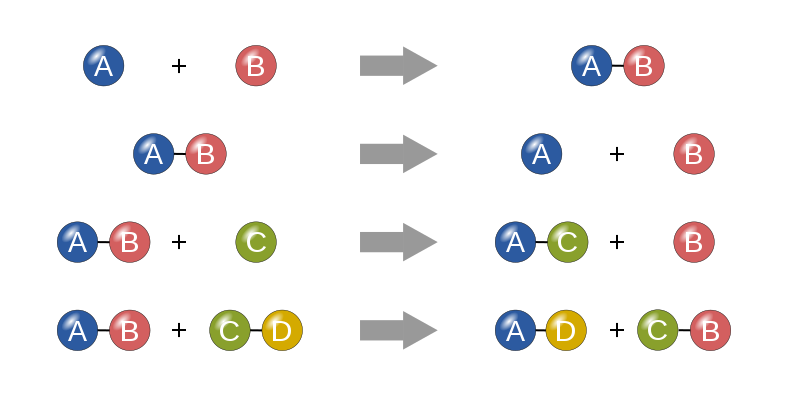
Figure 14: Chemical Change Examples
(Pugliesi, 2012)
The difference between a chemical change and a physical change is that a chemical change changes the chemical identity (arrangement of atoms) of the substance. A physical change occurs without changing the chemical identity. The chemical properties of atoms determine the physical properties we see.
Chemical change
Causes a new substance to be formed.
Examples of Chemical Change
- Rusting
- Combustion
- Baking a cake
- Color change
Physical change
Can be observed or measured without changing the identity of the substance.
Examples of Physical Change
-
Melting
-
Boiling
-
Freezing
Chemical and Physical Change
What are the differences between chemical and physical change? Watch this video to learn more:
Teacher Time Out: Chemical Reactions
How does this connect to the NGSS Progression
DCI: PS1.B Chemical Reactions: In K-2 students can observe that heating or cooling a substance may cause changes that are reversible or not. As students progress to 3-5, The focus is on mixing different substances and the different properties that may be formed even though the total weight of the substance does not change. When students enter middle school (6-8) they begin to focus on the chemical process of the items from the original substance regrouping into different molecules and the new substances that have differing properties.
|
K-2 |
3-5 |
6-8 |
|
| PS1.B Chemical reactions | Heating and cooling substances cause changes that are sometimes reversible and sometimes not. | Chemical reactions that occur when substances are mixed can be identified by the emergence of substances with different properties; the total mass remains the same. | Reacting substances rearrange to form different molecules, but the number of atoms is conserved. Some reactions release energy and others absorb energy. |
https://static.nsta.org/ngss/20130509/AppendixE-DCIProgressionsWithinNGSS_1.pdf
Implementation into the Classroom
Baking Soda Balloons- Have students mix baking soda and vinegar together. As the chemical reaction is occurring have them capture the gas in a balloon. Ask students if any matter was created or destroyed. Many students may think that matter was created because the balloon expanded. Help students gather more information on this concept by having them do the activity again. This time have them weigh all the materials to see if anything was destroyed.
Slime Time– Students can create slime by measuring out all the different ingredients. As they mix everything together help students recognize that, though they have a new product, the overall weight stayed the same.

