5 The Basic Unit
The structure of all items on Earth lead to specific or multiple functions that relate to how items can interact. These interactions can be predicted by observing varying patterns across the matter found on Earth. As you work through this section and investigate matter further you will be able to gather a deeper understanding of the big ideas and start to answer some of the big questions.
Big Ideas and Big Questions
Big Ideas
- Matter is made up of small particles called atoms.
- Because different kinds of matter are made of different arrangements and types of atoms, different kinds of matter have different properties
Big Questions
- How does the structure of atoms affect the properties of matter?
Matter
All things are made of matter, and all types of matter have different characteristics that can be observed through our senses or enhancement of our senses. Matter is composed through the arrangements of atoms in compounds, mixtures, and elements. These three categories describe the makeup and characteristics of all things. For example, water is a compound. The makeup of water is explained through the arrangement and bonding of the atoms hydrogen and oxygen. Water is composed of hydrogen and oxygen resulting in the arrangement of a compound.
The arrangement of atoms helps define the behaviors of the atoms. The behavior of the atoms define the states of matter of a solid, liquid, and gas. The behaviors can be looked at as characteristics (figure 6). Referring back to the water example, one characteristic of water can be what happens when the temperature of water changes. Water in its original form is a liquid. If water gets heated up it can start to boil and be released as steam which is a gas. If water gets below freezing the water freezes and turns into ice becoming a solid. In the process of changing the state of matter the characteristics continue to change under the surface in the movement of the molecules. This is just one example of how the characteristics of matter are dependent upon the chemical makeup of the matter interacting with the environment around the matter.
Classification of Matter – Arrangements of Atoms
Element
Compound
Mixture
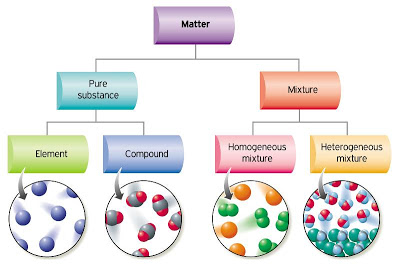
Figure 6: Arrangement of Matter
(Lojaya, 2013)
Teacher Time Out: Matter, Atoms, Element, and the Periodic Table
How does this connect to the NGSS Progression
DCI: PS1.A Structure of Matter: Students focus on the structure and functions of matter beginning in K-2 with a variety of objects that are made up of smaller pieces that can be seen without the enhancement of the senses, and advancing across the grade bands (3-5 → 6-8) to incorporate structures of matter that cannot be seen without enhancement of the senses. Learning about the chemistry concepts such as matter, atoms, elements, and the periodic table allow us to be equipped to implement these learning concepts for our students in our future classrooms.
|
|
K-2 |
3-5 |
6-8 |
| PS1.A Structure of matter (includes PS1.C Nuclear processes) | Matter exists as different substances that have observable different properties. Different properties are suited to different purposes. Objects can be built up from smaller parts. | Because matter exists as particles that are too small to see, matter is always conserved even if it seems to disappear. Measurements of a variety of observable properties can be used to identify particular materials. | The fact that matter is composed of atoms and molecules can be used to explain the properties of substances, diversity of materials, states of matter, phase changes, and conservation of matter. |
Fromhttps://static.nsta.org/ngss/20130509/AppendixE-DCIProgressionsWithinNGSS_1.pdf
Implementation into the Classroom
An initial engagement experience into chemistry concepts
An investigation where students observe the relationship of subatomic particles, atoms, and the periodic table
Atoms
Atoms compose all matter and are the smallest unit of matter. The different compositions of different types of matter have to do with the specific structure and make up of the specific atoms in a particular type of matter. Specifically it has to do with the different types of atoms, arrangements of atoms, and movements of atoms. These factors influence why everything is different. If it was not for the variety of types of atoms everything would be composed the same way.
Atoms are tiny particles that are composed of subatomic particles. The subatomic particles of an atom are protons, neutrons, and electrons. Protons are positively charged located inside the nucleus. Neutrons are neutral; they are not positive or negative. Neutrons are also located inside the nucleus. Electrons are negatively charged and are located outside of the nucleus. For more information refer to figure 7 and 8.
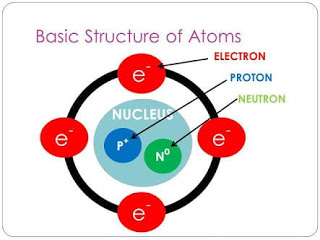
Figure 7: Structure of an Atom
(Ghaniscoach, 2019)
Subatomic Particles
Protons
Neutrons
Electrons
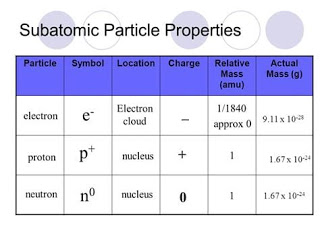
Figure 8: Subatomic Particle Properties
(Ghaniscoach, 2019)
Explore Subatomic Particles – Types of Atoms
You can explore the types of atoms in this SIMULATION.
When interacting with the simulation:
- What does the model show?
- What Elements can you build? How many Protons, Neutrons, and Electrons are needed for a particular element?
- What does adding/removing Protons, Neutrons, and Electrons achieve?
- What happens to the mass number? net charge?
- Describe the differences and similarities between the two models; cloud and orbits.
- What happens when you change the show (e.g. Element, Neutral/Ion, and Stable/Unstable)?
Periodic Table
The periodic table (figure 9) consists of all of the atoms in the universe. The atoms on the periodic table are elements. An element is a pure substance and there might be lots and lots of atoms in a sample of an element, but they are all the same type of atom. Any sample of the elements on the periodic table is an example of what would be classified as an element. Within each square on the periodic table you will find the element symbol, atomic number, and atomic mass. The atomic mass reported is the average atomic mass of the specific type of atom. The atomic number is the total number of protons. The periodic table is arranged by the atomic number. Typically the table shows a color code indicating the elements as nonmetals and metals which can be further broken into categories. On the periodic table you can also see the elements arranged in groups (number above 1-18) and periods (number on the sides 1-7 periods). For more information about the periodic table see the videos and interactive below.
To learn more about the Periodic Table
Watch these videos
https://www.yout-ube.com/watch?v=t_f8bB1kf6M
https://www.yout-ube.com/watch?v=0RRVV4Diomg
Interactive
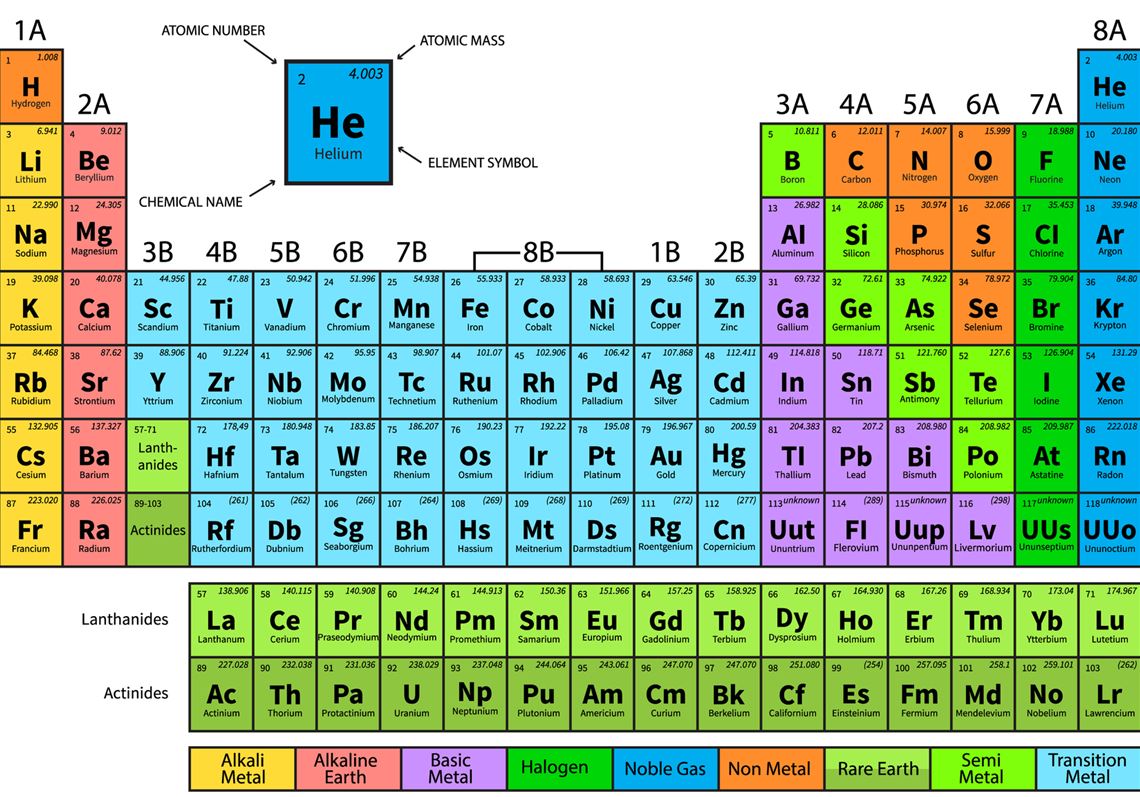
Figure 9: The Periodic Table
(Shribman, 2019)
Reflect
- How do these concepts apply to lab activities?
- Can you relate the concepts to the Big Ideas?
- How can you apply these concepts in your future classroom?

