II. Chemical Foundations
Rate of Reaction
For the reaction:
aA + bB → cC + dD
the rate of the reaction can be expressed as,
rate = k[A]x[B]y
where k is the rate constant and x and y are the orders of reaction (powers) with respect to the concentrations of A and B respectively. The concentrations that appear in this equation are not limited only to reactants but can include any chemical species that impacts the rate, such as a catalyst or inhibitor.
To make this concrete, consider the reaction that you will focus on in the laboratory portion of this topic, the decomposition of hydrogen peroxide:
![]()
In this reaction, as in most reactions, reactants must overcome a potential energy barrier to become products. This barrier is called the activation energy. In this case, the barrier might be imagined to be a state in which the oxygen-hydrogen bonds of one hydrogen peroxide molecule are being stretched (broken) as oxygen-hydrogen bonds on an adjacent molecule are being formed. The course of the reaction might be pictured as the figure below.
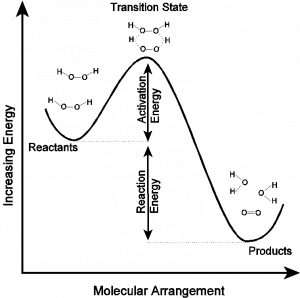
In this potential energy diagram, the difference in energy between the products and reactants is related to the thermodynamic spontaneity of the reaction. (You will learn more about this in Chapter 19). However, it is not the spontaneity but rather the magnitude of the activation barrier that has the largest influence on the rate of the reaction. The molecular arrangement with the highest energy along the path from reactants to products is called the transition state. As the name indicates, this state defines the transition between reactants and products, and it only occurs with a fleeting existence (i.e. 10-15 sec) as the reactants undergo the transformation to products. Based on this diagram, it is reasonable to expect that the decomposition will release energy but will be slowed due to the substantial activation energy (Ea= 76 kJ/mol).
To traverse the path from reactants to products, molecules must have enough thermal energy to surmount the activation barrier. At any given temperature (other than absolute zero), molecules have a distribution of energy. That is to say, some molecules have a higher instantaneous energy than other molecules. The average amount of thermal energy that molecules possess at any given temperature is on the order of RT (at room temperature, RT=8.3 J mol–1 K–1 x 298 K = 2.5 KJ/mol). The fraction of the molecules that have enough energy to overcome a potential energy barrier of magnitude Ea depends exponentially on the ratio of the activation energy to the thermal energy. Mathematically, this takes the form of the Arrhenius equation,
k = Ae-Ea/RT
where k is the rate constant of the reaction, A is a constant that depends on how frequently the reactants are found in the proper configuration to react, and the exponential term expresses the fraction of the molecules that have enough thermal energy to overcome the activation barrier. Given this mathematical form, two strategies for increasing the rate of the reaction become apparent. All other things being equal, (1) the rate of the reaction will increase exponentially with increasing temperature, and (2) the rate of the reaction will increase exponentially with decreasing activation energy. The first strategy is straightforward to implement by simply heating the reaction system. The second method, decreasing the activation energy, can be achieved by adding a catalyst to the system.
Catalysts
A catalyst is a substance that can increase the rate of a reaction while not being consumed. Catalysts usually function by breaking the reaction up into a sequence of steps with an overall reduction in the activation energy and thus overall enhancement of the rate. A possible reaction diagram for a catalyst is given below.
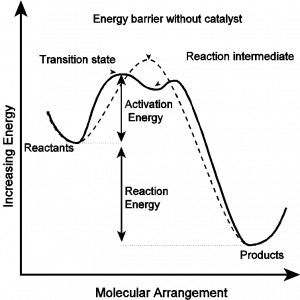
It is useful to define three categories of catalysts: (1) Homogeneous catalysts are chemicals that are in the same phase as the reactants; (2) Heterogeneous catalysts are chemicals that are in a different phase from the reactants; and (3) Enzymes are polypeptides (proteins) capable of catalyzing reactions. You will be studying examples from each of these categories.
In the presence of a catalyst, the reaction rate of hydrogen peroxide decomposition can be enhanced substantially. First, consider the homogeneous catalytic decomposition of hydrogen peroxide by iodide ion (I–). The elementary steps and relative rates are given by,
Step 1. ![]() SLOW
SLOW
Step 2. ![]() FAST
FAST
overall ![]()
(What would be the expected rate law for this mechanism? Why? Answer: rate = k[H2O2][ I–]. The rate law is written for the slow (rate-determining step)). Catalysis by iodide is able to promote the speedy formation of oxygen and water largely due to the reduction in the activation energy to about 57 kJ/mol (compared to 76 kJ/mol for the uncatalyzed reaction).
Heterogeneous catalysis is also possible for the hydrogen peroxide decomposition reaction. Manganese(IV) oxide (MnO2) is a black solid that is insoluble in water. Since the solid is insoluble, any enhancement of the rate of decomposition must occur through a heterogeneous pathway. Because the reaction takes place at the solid/liquid interface, one of the problems that must be addressed experimentally is that molecules must be continuously transported to the interfacial region. For instance, if a layer of O2 gas forms on the solid, new hydrogen peroxide molecules, which are in the solution phase, cannot reach the active surface in order to react. Continuous stirring is one simple solution that works in this case. One of the most significant advantages of heterogeneous catalysts is that since they are present as a separate phase they can easily be separated from the reaction mixture and recycled.
A special form of homogenous catalysis that you will investigate will be enzyme catalysis. Hydrogen peroxide is a strong oxidant, and therefore can cause serious molecular damage to cells. Hydrogen peroxide is known to readily react with unsaturated fatty acids in cell membranes causing damage to the membrane structure (thus its use as a disinfectant). To protect the cell against oxidative damage, cells produce enzymes that can accelerate the decomposition of hydrogen peroxide into the less harmful molecules: oxygen and water. One enzyme that has evolved to serve this function is catalase.
Catalase is an iron-heme containing enzyme (a picture of a heme group is given in experiment 10. In topic 10, you will be studying another heme protein, hemoglobin). A heme group is a large cyclic molecule with a pocket in the center that is capable of binding metal ions. Catalase is a tetramer (made up of four parts), with a total molecular weight of about 250,000 g/mol. Each of the tetrameric subunits contains one “active site” composed of a heme-bound iron(III) surrounded by protein. This active site is the location where H2O2 binds and decomposes to oxygen and water. Structures of catalase from several sources have been obtained, and one structure of catalase from bovine liver obtained by I. Fita and M.G. Rossmann is presented as a ribbon representation below.
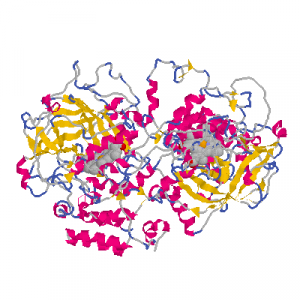
Although simple iron salts [e.g. Fe2(SO4)3] possess some catalytic activity for the decomposition of hydrogen peroxide, further enhancement is achieved by the binding of the iron within the heme of the enzyme. Catalase is a very efficient enzyme, and in the cell, it is able to decompose hydrogen peroxide nearly as fast as hydrogen peroxide can reach the enzyme. All three components of the enzyme, the iron(III) ion, the heme group, and the protein are essential to proper catalytic function of catalase.
Because all three components are vital to catalytic function, the disruption of any one of the components can inhibit catalysis. An inhibitor is a chemical species that slows the rate of the reaction. In the case of catalase, copper(II) ions can function as an inhibitor. Copper(II) is thought to compete with the iron for the heme binding site. If a copper(II) ion is bound in the heme pocket, the enzyme does not function as a catalyst, and therefore the rate decreases.

