3 V. Laboratory Activity
Preparation
- Know Unit 4 Module 3 of your textbook (and ΔHrxn from Unit 5 Module 2)
- Read all of lab Topic 7
- Complete pre-lab questions before coming to lab
- Prepare for statistical analysis be reading Appendix 1
Do this before you come to lab (have at the top of your notebook page to begin the lab):
- Safety: Answer the following questions:
- What precautions should be considered for safely handling dodecane burner during the lab activity? Especially what should you think about the environment near the burner before you use a lighter to ignite?
- What should you do if you accidently inhale too much gas based on SDS instruction? How would you know if you have inhaled too much?
- Purpose: Write a few sentences about the purpose of this lab: What is the “big picture” viewpoint of the lab experiment? What question am I trying to answer? What data will be collected to answer the questions? What technique will I use to obtain these data?
- Preparation: It is important to know how to experimentally determine the energy density of an unknown sample. Using dodecane, the calorimeter constant was found to be 28.9 kJ/°C, calculate the amount of kilocalories (Cal) per gram given off by a unknown sample if burning 0.5 g caused the temperature of the calorimeter to rise from and initial temperature of 25.0 °C to 30.5 °C. (Reminder: food labels use calories (Cal); 1 Cal = 1000 cal; 1 cal = 4.184 J)
A. Introduction
Your body can be compared to an engine. In order for an engine to run, it must have a source of fuel in an atmosphere that supports combustion. The fuel that your body uses is the food you eat and the oxygen that you breathe supports the combustion of that food. Different kinds of food generate different amounts of energy. The major food groups, proteins, carbohydrates, and fats, are composed mainly of compounds that contain carbon, hydrogen, and oxygen. Therefore, the main byproducts of metabolism of the food in our bodies are carbon dioxide, water, and energy. Because the heat energy known as enthalpy given off by a reaction (at constant pressure) is a state function, its value only depends on the initial and final conditions. Therefore, any process that starts with food and ends up with carbon dioxide, water, and energy can be used to measure the amount of food energy released as enthalpy. This is the key concept that will be used to define the experimental strategy of this laboratory experiment. Food energy can be measured by measuring the amount of heat released when food samples are burned by a flame.
Of course the next question is, “How can heat energy be measured?” One answer is through the use of a calorimeter. A calorimeter is a device used to measure the heat energy produced in a chemical reaction using temperature measurements. Since you are interested in a quantitative measurement and initially the relationship between heat and temperature is unknown, you must determine this relationship experimentally through calibration. In addition, by carrying out the calibration experiment several times, you will also be able to estimate the precision of the method.
In this experiment, the energy of various foods will be measured using the same general technique that is used to collect the data required for packaging. The foods will be burned in the laboratory using a simple calorimeter. The burning food will be used as the fuel to heat a calorimeter containing a measured amount of water. From the amount of fuel and temperature change of the calorimeter, the amount of energy generated by the fuel source can be calculated.
B. Your Laboratory Challenge-Measuring Food Energy
Part 1: Calibration (Teams of 2 people per computer):

- A simple calorimeter will be constructed using a 250 mL Erlenmeyer flask held by a buret clamp. A thermocouple temperature probe will be attached to a computer interface and will be used to measure temperature. A dodecane burner will be used to generate a known amount of heat. (See photo above). If you have any questions regarding the setup, be sure to ask your TA
- You will be using Logger Pro software to monitor the temperature as a function of time during the experiment. To navigate to the appropriate file, select the following folders:
- To calibrate your calorimeter, you will use dodecane, an alkane hydrocarbon with a heat of combustion that is known from your pre-lab activity and the NIST-web site. The basic procedure is as follows. Using an analytical balance, determine the mass of your dodecane burner (remember to zero the balance before any reading). Set up your calorimeter by measuring 50 mL of room temperature water into the Erlenmeyer and assembling as pictured above. Start the data acquisition by clicking on the
 button above the graphing portion of the screen. After about 15 seconds, light the burner under the flask with a lighter. Be sure to remove the lighter as soon as the sample is ignited so the heat generated by the lighter is not measured by the calorimeter. Allow the flame to heat the system until a temperature rise of approximately 10˚C is observed. At this point, extinguish the flame and continue to stir and monitor the temperature for an additional 30 seconds. Finally, re-determine the mass of the dodecane burner. Remember, you should directly record all measurements, such as mass readings, in your laboratory notebook (use a table format).
button above the graphing portion of the screen. After about 15 seconds, light the burner under the flask with a lighter. Be sure to remove the lighter as soon as the sample is ignited so the heat generated by the lighter is not measured by the calorimeter. Allow the flame to heat the system until a temperature rise of approximately 10˚C is observed. At this point, extinguish the flame and continue to stir and monitor the temperature for an additional 30 seconds. Finally, re-determine the mass of the dodecane burner. Remember, you should directly record all measurements, such as mass readings, in your laboratory notebook (use a table format). - Using the graphical analysis software, you can determine the initial and final temperature using the statistics function in the following way. Highlight a period either at the start or at the end of the experimental trial that is relatively constant in temperature. Then click on the
 button to return the average value of the points within the selected region. Record your initial and final temperatures in your laboratory notebook.
button to return the average value of the points within the selected region. Record your initial and final temperatures in your laboratory notebook. - Repeat steps 3 and 4 for three calibration runs. Note: the water should be exchanged for each determination.
Part 2: Food Energy Measurement (Teams of 2 people per computer):

- Each team will be asked to measure one type of food product three different times. Your TA will tell you which food your team will measure (Enter the name food item in your lab notebook). The procedure for making the measurement will be analogous to the one used to calibrate your calorimeter, except that solid food products will be held by a wire, available in the lab (See photo above). Be sure to remove the lighter as soon as the sample is ignited so the heat generated by the lighter is not measured by the calorimeter.
- Similar to your calibration steps, allow the flame to heat the system until a temperature rise of approximately 10˚C is observed.
- Be sure that all of your measurements are accurate and that the balance you use is zeroed before each reading. Carry out the measurement as you did for the dodecane, being sure to record the initial and final temperatures of your calorimeter.
- For increased accuracy, measure the heat generated by each food three times. Note: the water should be exchanged and a new sample used for each determination.
- There is no hazardous waste generated during this experiment. Solid waste can be disposed of in the waste basket; however, please make sure that the sample is fully extinguished and cool.
Key equations and ideas:
- Remember the heat of an exothermic reaction is negative.
- Heat = mass times heat of combustion
![]() (in energy/mass units)
(in energy/mass units)
- Heat of reaction = – heat capacity times the temperature change
![]() (also,
(also, ![]() )
)
- Fuel value = – heat of combustion (often in units of Cal/g)
C. Communicating Your Results
For a permanent record, both your data and report should be written in your notebook.
- Safety
- Purpose
- Preparation
- Provide a summary of your temperature and mass change data in clear and well organized tables.
- From the known value for the heat of combustion of dodecane (starting in units of kJ/mol), calculate the heat of combustion of dodecane in units of Cal/g.
- Use the mass of the dodecane consumed to calculate the heat produced in each calibration trial. (in units of Cal). Show one sample calculation.
- Use the calculated heat and the observed temperature change for each calibration trial to determine the calorimeter constant of your calorimeter (Show one sample calculation). Determine the mean of all three of your values.
- Use your mean calorimeter constant to determine the heat produced in the food measurement trials (in units of Cal) (Show one sample calculation). Then use the mass losses to determine the fuel values of each trial (in units of Cal/g) (Show one sample calculation). Determine the mean and standard deviation of your results.
- Finally, compare your experimental values to the values reported in the “Nutritional Facts” table on the food package. From the Appendix 1 on statistics in Topic 7, you should recall that for 3 measurements subject to random errors, it can be stated that the “true value” should lie within
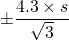 of the mean value (s=standard deviation, you can use excel to calculate) with 95% confidence.
of the mean value (s=standard deviation, you can use excel to calculate) with 95% confidence.
- Calculate and report the 95% confidence interval based on your data. Does the value from the label lie within this range?
- Comment on the consistency of your results with the value published on the label.
- Write a brief conclusion paragraph: comment on whether or not you achieved the goal of the experiment, respond to the questions posed in the introduction. Support your conclusion using your data.

