7
Formative Assessment
When water gets cold, it becomes ice. As a candle burns, solid wax turns into a liquid. Iron rusts. The matter around us is always changing, and these changes are critical for life on Earth. In this chapter, we explore how matter changes as a result of two different processes:
- Heating and cooling
- Mixing substances
Heating and cooling can cause matter to expand, contract, or to change its state. Mixing substances can result in a chemical reaction in which new substances are formed. The best way to understand and explain these changes in matter is through the particle model of matter.
Changes during heating and cooling
Expansion and contraction
The temperature of a substance measures how quickly its particles vibrate and/or move. When a substance is heated, particles move faster, which forces there to be more space between them.
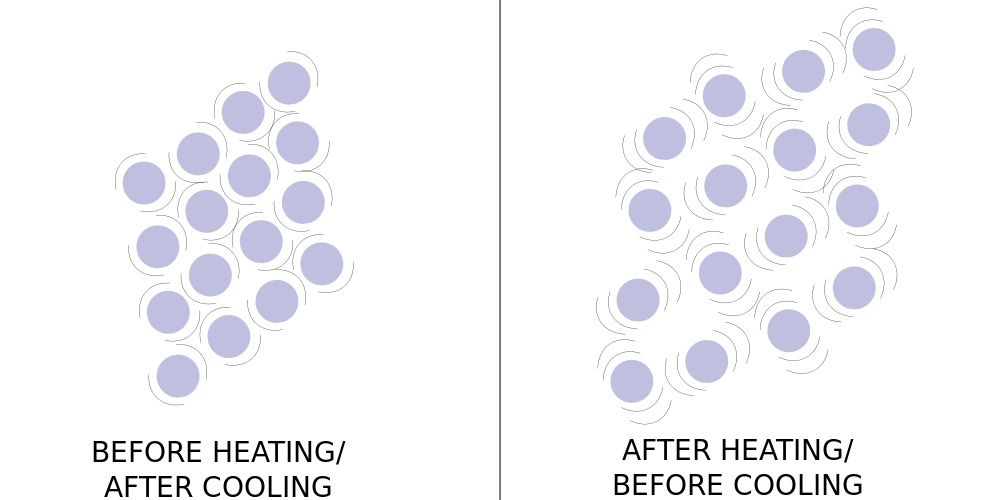
Macroscopically, we see this as the expansion of materials, for example, bulging and cracking in concrete as it tries to take up more space than is available.

Likewise, materials contract when cooled. This can also manifest as damage to macroscopic materials. One of the reasons why roads in Iowa are so filled with cracks is the constant cycle of expansion and contraction as the temperature can go from way too cold for comfort in the winter to way too hot for comfort in the summer.
Changes in the state of matter
There are three main states of matter: solid, liquid, gas. There are actually lots of others, and many have important roles in technological devices, but our everyday interactions with matter are dominated by matter in the form of solid, liquid, and gas. Elementary school students should therefore only investigate these states of matter.
Each state of matter corresponds to a particular way that particles are arranged.
In a solid, particles are rigidly held in place relative to their neighbors. They vibrate back and forth as they collide with – and are attracted to – their neighboring particles, but they don’t really move around relative to their neighbors. As a result, solids have a well-defined shape and volume.
In a liquid, particles generally move fast enough to overcome their attraction to their immediate neighbors, but not fast enough to escape the group. Particles are free to slip and slide past each other, but not to break free on their own. As a result, liquids have a specific volume, but a variable shape – if liquids are in a container, they will conform to its shape but keep their particular volume.
In a gas, particles are moving fast enough to break away from the group. They are largely zipping around on their own. This means that they keep going until they hit either another particle or the walls of the container – if they are in one. As a result, gases do not have a specific shape or volume – if gases are in a container, they will conform to both its shape and volume.
Matter changes between solid, liquid, and gas as a function of two things:
- Particle speed (which changes based upon temperature)
- Attraction between particles (which changes based upon the type of particles)
Some substances, like Hydrogen and Helium, have very low attraction between particles. As a result, they are always gases here on earth (outside of exotic laboratory conditions). Their particles simply don’t attract each other strongly enough to condense into a liquid (never mind a solid, which would require even stronger attraction!). Most of the time, we think of metals as being solid. But you’ve likely heard of Mercury, which is a metal that is a liquid at room temperature because its particles don’t attract each other as strongly as other metals do. One particularly interesting metal is called Gallium, which is composed of particles that attract just enough to be a solid at room temperature. But even human body temperature is high enough to make Gallium particles move fast enough to overcome their attraction to their neighbors and slip and slide past each other – becoming a liquid. Gallium melts in your hand!
When substances go from solid to liquid, we call it melting. From liquid to solid, we say freezing. Condensation is when a gas becomes a liquid, and boiling is when gas becomes a solid. But interestingly enough, substances can also go from solid directly to gas, and vice versa. You may have heard or even used “dry ice”. It’s actually made of carbon dioxide (CO2). In everyday conditions, carbon dioxide particles don’t form a liquid. That is, they don’t have the phase where they slip and slide past their neighbors – they are either tightly held in place, or they escape the group of particles entirely. Going from solid directly to gas is called sublimation. We call it “dry” ice because there is no liquid involved. Of course, it’s not really ice since there’s no water involved either. Oh, and by the way, it’s also possible to go directly from gas to solid – this is called deposition or desublimation. But elementary students should only study freezing/melting and boiling/condensing; that said, if they see dry ice, they’ll probably ask you about it.
Explore States of Matter – Behavior of atoms
You can explore the states of matter in this simulation.
When interacting with the simulation:
- What happens when you increase temperature? Decrease temperature?
- Describe the atoms and molecules in the different states of matter.
- What happens when you change the atom and molecule types (e.g. Neon, Water, etc.)?
Mechanisms of heating and cooling
Things can heat up or cool down in three different ways: conduction, convection, and radiation.
Conduction
In conduction, faster-moving particles in a hotter object collide with slower-moving particles in a colder object. Conduction transfers energy through direct particle collisions, as in this simulation:
When they collide, faster-moving particles speed up the slower-moving particles, and in the process, the faster-moving particles slow down. Conduction will continue until both objects reach the same temperature.
Convection
In convection, a region of space gets warmer as faster-moving particles move from one place to another. Particles need to be free to move in order for convection to happen, so it doesn’t happen in solid materials – only liquids and gases. One common way for convection to happen is unevenly heating a material. As particles in one region get warmer and begin to move faster, they spread out. This makes them less dense in that area. Well, less dense things float on more dense things – so they begin to rise. You’ve heard that “hot air rises”? This is why. As they rise, cooler particles fill the space below them (or, you could also think that gravity pulls the more dense material to the bottom, so they push the less dense material up). On Earth, the Sun unevenly heats the atmosphere and other materials, making hotter air rise and cooler air rush in to fill the space. And voila! Wind.
Radiation
Step out into the Sunlight, and you will experience heating via radiation. You can feel its warmth. Radiation happens because the particles that make up matter are electrically charged. This is why they attract each other in the first place (and repel if they get too close). Sunlight is a form of “electromagnetic radiation”, which means that it is made up of electric and magnetic fields that travel as waves through space. Just like a magnetic field can push or pull on a magnet, electric fields can push or pull on electrically-charged objects. When sunlight (electromagnetic radiation) hits the particles that make up your skin, the waves of electric fields pushes/pulls the charged particles in your skin, making them move faster. Think of a wave in water pushing a boat up and down – it’s the same thing except light has waves of electric field rather than water, and it moves charged particles instead of boats.
Changing matter by mixing substances
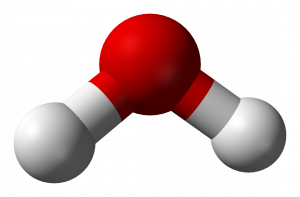
There are about 100 different types of atoms. But there are way more than 100 types of substances. These different substances exist because some atoms are really strongly attracted to other types of atoms. These strong attractions tend to make the atoms stick together in a collection called a molecule. This molecule behaves like a particle, because the atoms move and collide as a group. Two hydrogen and one oxygen atom makes a single molecule of water. One way to represent a water molecule is shown below, with the red ball representing oxygen (O) and the white balls representing hydrogen (H).
Methane is made of four hydrogen (H) and one carbon (C) atoms. A representation of this molecule is below, with the carbon atom shown in black and the hydrogen atoms again in white.

If different types of molecules are mixed so that different molecules collide with each other, and interesting thing can happen. The atoms may rearrange as they are more attracted to atoms in the other molecule than they are to the atoms in their own molecule. This rearrangement results in new molecules that then interact with other particles in new ways. We see this as different properties. We call this a chemical reaction.
If we mix baking soda and vinegar, the atoms in vinegar and the atoms in baking soda rearrange forming new molecules. One of those new molecules is carbon dioxide, which isn’t so attracted to other particles, so it is gas at room temperature. We see this carbon dioxide as bubbles.
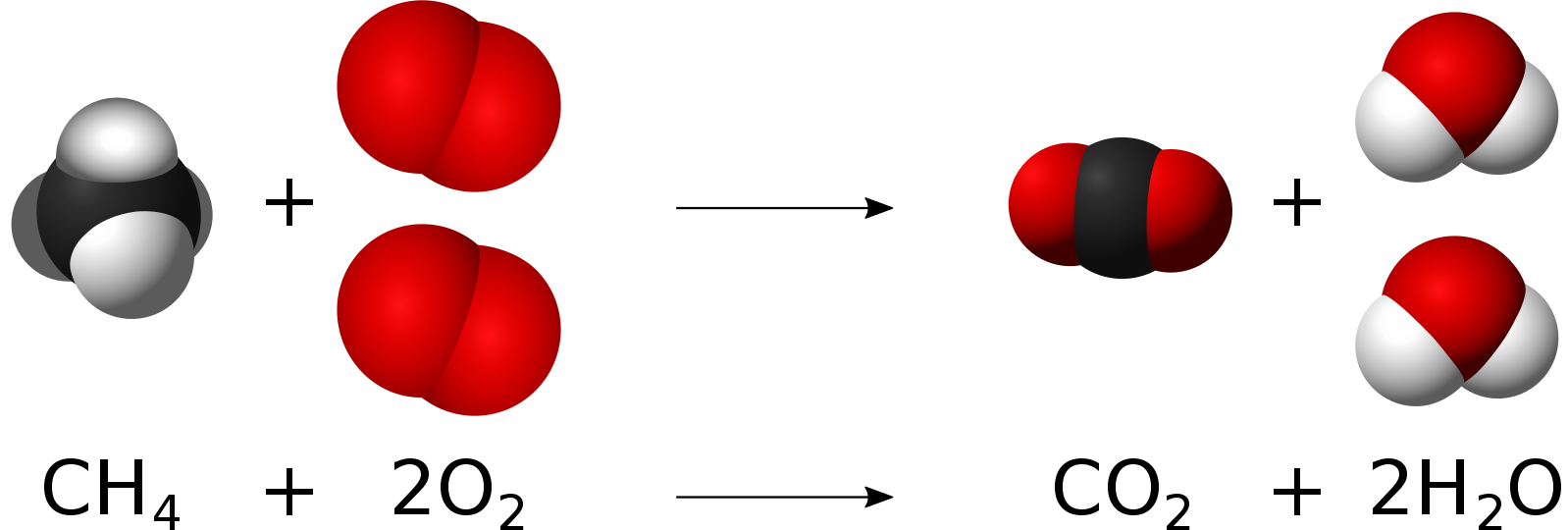
Often, molecules don’t rearrange unless they are moving so fast that the collisions smashes atoms loose from their original molecule. If molecules of methane and oxygen are moving fast enough, the atoms making up each type of molecule will be separated, and they will recombine as carbon dioxide and water. As these atoms rearrange, they really speed toward each other due to strong attractions, so the newly-formed molecules end up going even faster than before. This means they are even hotter than before! We call this burning, or combustion.
In the simulation below, notice how:
- Particles of fuel and oxygen molecules need to be moving fast enough in order to recombine
- Newly formed molecules (exhaust) typically go flying off really fast after they are formed
No matter the chemical reaction, atoms simply rearrange – they are neither created nor destroyed. In the methane and oxygen reaction above, there is one carbon atom, 4 hydrogen atoms, and 4 oxygen atoms before the atoms rearrange; and there is one carbon atom, 4 hydrogen atoms, and 4 oxygen atoms after the atoms rearrange. No matter how substances react during a chemical reaction, there is as much matter before the reaction is the same as there is afterwards. That is, matter is conserved.
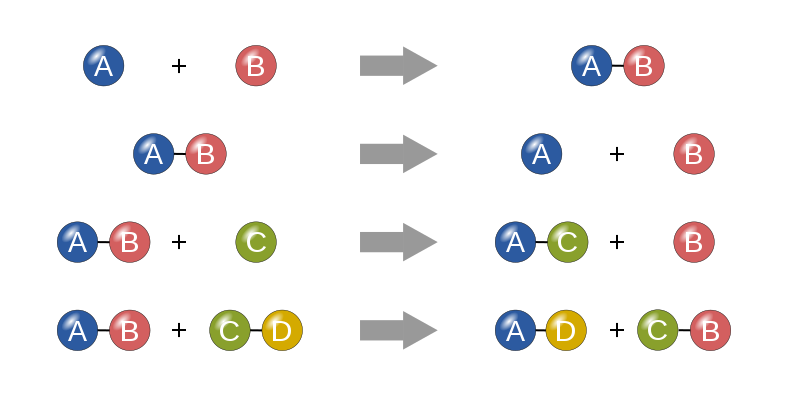
The figure below shows models of different types of chemical reactions. In each one, there are as many atoms on the left of the arrow as on the right.
NGSS Performance Expectations
| 2-PS1-4 | Construct an argument with evidence that some changes caused by heating or cooling can be reversed and some cannot. [Clarification Statement: Examples of reversible changes could include materials such as water and butter at different temperatures. Examples of irreversible changes could include cooking an egg, freezing a plant leaf, and heating paper.] |
|---|
| 5-PS1-4 | Induct an investigation to determine whether the mixing up two or more substances results in new substances. |
|---|
Disciplinary Core Ideas
PS1.B: Chemical Reactions
- Heating or cooling a substance may cause changes that can be observed. Sometimes these changes are reversible, and sometimes they are not.
- When two or more different substances are mixed, a new substance with different properties may be formed.

