5
Formative Assessment
Water flows; ketchup flows slowly. Diamonds are hard; chewing gum is soft. Everything around us can be described in terms of its properties. Think of something that is transparent, hard, and brittle (cracks instead of bending)? If you are thinking of glass or crystal, you’re not alone. Not only can an object or material be described in terms of its properties, but knowing the properties of an object or material can help you guess what it is.
Big Ideas in This Chapter
Big Ideas
- Objects and materials have a distinct set of properties.
- Properties of objects can help us identify the materials they are made from.
What properties matter?
Which piece is really gold?


Which is the real diamond?


How can we tell?
King Hiero II of the Ancient Greek city of Syracuse had a problem. He had commissioned a magnificent crown made of pure gold from a goldsmith. He delivered an exact amount of gold to the goldsmith in order for him to construct the crown. Many days later, the goldsmith delivered the crown to King Hiero. It was indeed magnificent. And equally importantly, the crown weighed exactly the same as the amount of gold that Hiero had given the goldsmith. He felt satisfied and proud of the magnificent crown.
Then, rumors began to reach him that he had been cheated by the goldsmith. Apparently, as the goldsmith was constructing the crown, he mixed in silver (a less valuable metal) with the gold as he constructed the crown, taking the leftover gold for himself. But the king was not sure. The crown looked and felt like gold. The crown was magnificent and pleased the king, but he was not sure whether he had been cheated.
He sent for a young citizen named Archimedes, who had gained a reputation for his investigations in mathematics and the natural world, and asked him to determine whether the crown was made of pure gold. Of course, Archimedes must not destroy the crown in the process!
According to legend, it was in the bathtub that the solution came to Archimedes. He noticed that as he got into the bathtub, his body pushed water out of the way, which then overflowed onto the floor. The amount of water that overflowed was exactly the volume of his body. He realized that if he put the crown into water, it too would displace water. He decided that he could put the crown into water and do the same with another piece of gold of the same exact weight that was known to be pure. If the same weight of metal displaced different amounts of water, then they must be made of different materials! The story goes that Archimedes was so excited about this revelation that he leapt from the bath, running through the streets naked yelling “Eureka!” This apparently translates to “I found it!” And thus the “eureka moment” was born.
Whether or not the story is fully accurate, it illustrates an important fact about the properties of materials. That is – if two objects are made from the same material, then they must have the same set of characteristic properties.
Properties of materials (or characteristic properties)
Characteristic properties are properties of materials. They do not change no matter how much or how little of the material you have. Iron is attracted to magnets no matter whether you have a big piece or a little piece. Copper conducts electricity whether it is in the shape of a wire or a penny. Water boils at 100 °C (212 °F) whether you have a small cup of it or a large stock pot. Characteristic properties are important for identifying whether two materials are the same. These include:
- Ability to conduct electricity
- Attraction to magnets
- Hardness
- Malleability (ability to be bend and shaped)
- Thermal conductivity (how readily heat is transferred through a material)
- Melting point
- Boiling point
- Density
To test whether King Hiero’s crown was made of pure gold, Archimedes used the last property on the list above: density. The density of a material is a measure of how heavy it is for its size. Whether you thought about it or not, you may have used the idea of density before to shop for fruit or to assess how filled an opaque bottle is.
Density
A good way to find a juicy orange is to pick it up. Is it heavy for its size? Then there is probably a lot of juice in there. Is it light for its size? That orange is probably pretty dry inside. If you pick up a bottle of shampoo and it is light, there is not a lot of stuff in there; if it’s heavy, the bottle is likely filled with shampoo. The density of an object is a measure of how much stuff there is in a certain amount of space. For two identical shampoo bottles, the heavier one has more stuff in the same space – so it is more dense. For two similar-sized oranges, the heavier one has more stuff inside – a good indication of juice.
Density tells you how much stuff (mass) is in a certain amount of space (volume). We can measure density by measuring the mass of an object and dividing by its volume, like this:
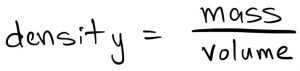
We measure mass in units of grams or kilograms. The most common way to measure volume (internationally at least) is in terms of milliliters or liters. Thus, two common units for density are grams per milliliter (g/mL) or kilograms per liter (kg/L).
Different substances have different densities. Water, for example, has a density of 1.0 g/mL, or 1.0 kg/L. Wow! What are the odds that the density of water – the most important liquid on Earth – would have a density of exactly 1.0 kilograms per liter? Quite good, actually. It’s not a coincidence. In fact, when scientists were constructing the metric system in the first place, they decided to that one kilogram would be the weight of 1 liter of water.
It turns out that whether or not something floats in water depends upon its density. If something is more dense than water, it sinks. If it is less dense, it floats. This can lead to some pretty interesting phenomena, like the one in this video:
Other characteristic properties
Density is a very useful way to tell materials apart. Archimedes used the fact that different metals have different densities to test whether a crown is made of pure gold. But there are lots of other characteristic properties (see the list above).
Have you ever heard that the alcohol “cooks off” if you use wine to make your pasta sauce? This is because alcohol has a lower boiling point than water (by the way, whether all of the alcohol boils off depends – of course – on how hot you get the dish and how long you cook it).
You might have heard that copper is an excellent electrical conductor. It is. Rubber is a bad one. So we can wrap copper wire in a rubber coating to make sure we don’t get an electric shock when handing a power cord. We can capitalize on the characteristic properties of materials to design and build different things that we need.
We can also use the characteristic properties of materials to determine whether what something is made of. Want to know whether something is a real diamond? Well, if you can scratch it with a metal nail, it’s not. Diamonds look a lot like a synthetic substance called cubic zirconia, but these two materials have different hardness and density (as well as other characteristic properties), making it straightforward to tell one from another.
Importantly, the characteristic properties of materials stay the same no matter how much you have. While the mass and volume are different for a small piece of chalk and a large piece of chalk, their density stays the same, since it is a characteristic property of the material, not a property of the object.
Properties of objects (or variable properties)
Above, we discussed how we can describe and identify materials using their characteristic properties. We can also describe objects in terms of properties. For example, an object has a particular mass or volume. These properties might be good for identifying different objects, but they are not good for telling materials apart, since they change depending upon the amount of material that you have. Likewise, properties like temperature don’t do much to tell materials apart since the temperature of an object can change depending upon its conditions. When describing properties of objects and materials, it is important to distinguish between properties that describe materials and properties that describe objects.
Knowing something about the properties of materials and objects helps us to use matter to design solutions to everyday problems, to determine what things are made of, and to more fully describe the matter than we see in the world around us.
Key Takeaways
- Materials have a set of characteristic properties, which do not change, no matter how much of the material there is. These properties include:
- Melting/boiling point
- Hardness
- Density
- Thermal/electrical conductivity
- Response to magnets
- Properties such as mass, volume, and temperature are properties of objects, and vary depending upon conditions.
- Characteristic properties of materials can be used to determine what things are made of
- Knowing the characteristic properties of materials allows us to use materials to design devices that behave in predictable ways and solve human challenges.
Performance Expectations
| 2-PS1-1 | Plan and conduct an investigation to describe and classify different kinds of materials by their observable properties. [Clarification Statement: Observations could include color, texture, hardness, and flexibility. Patterns could include the similar properties that different materials share.] |
|---|
| 2-PS1-2 | Analyze data obtained from testing different materials to determine which materials have the properties that are best suited for an intended purpose.* [Clarification Statement: Examples of properties could include, strength, flexibility, hardness, texture, and absorbency.] [Assessment Boundary: Assessment of quantitative measurements is limited to length.] |
|---|
| 5-PS1-3 | Make observations and measurements to identify materials based on their properties. [Clarification Statement: Examples of materials to be identified could include baking soda and other powders, metals, minerals, and liquids. Examples of properties could include color, hardness, reflectivity, electrical conductivity, thermal conductivity, response to magnetic forces, and solubility; density is not intended as an identifiable property.] [Assessment Boundary: Assessment does not include density or distinguishing mass and weight.] |
|---|
Disciplinary Core Ideas
PS1.A: Structure and Properties of Matter
- Different kinds of matter exist and many of them can be either solid or liquid, depending on temperature. Matter can be described and classified by its observable properties.
- Different properties are suited to different purposes.
- Measurements of a variety of properties can be used to identify materials. (Boundary: At this grade level, mass and weight are not distinguished, and no attempt is made to define the unseen particles or explain the atomic-scale mechanism of evaporation and condensation.)
Crosscutting Concepts
Patterns
Scale, Proportion, and Quantity
- Standard units are used to measure and describe physical quantities such as weight, time, temperature, and volume.

