6
Formative Assessment
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.
Richard Feynman, Nobel-prize winning physicist
The idea that matter is composed of atoms is perhaps the single most important idea in all of science. This tiny idea can be used to explain a truly astonishing number of phenomena and observations that elementary students regularly notice and wonder about, such as:
- Why does glass shatter but rubber snaps back into shape?
- Why don’t oil and water mix?
- Why can I see my breath when it’s cold outside?
- Why are some things liquid and other things solid?
Of course, the idea that matter is composed of atoms is far from obvious. Atoms are so small that no human ever has, or ever will, see one directly. So why did people start thinking atoms exist in the first place? Good question – this wasn’t always the case.
A little history
The Greeks thought about a lot of stuff. One of those Greeks was named Empedocles. Though you might not know his name, you might be familiar with his idea that there are four basic elements (though he did use this term): earth, water, air, and fire. In hindsight, we know this is not the case, but when Empedocles lived, his thinking was a revolution, and his idea was considered fact for about two thousand years. So why was it a good idea? Because thinking of all the things around us as composed of combinations of these four basic elements can lead to some uncanny explanations of what we observe.
In Empedocles’ model of matter, each of the four elements have a rightful place in the world. Earth – in its purest form – belongs to the Earth. So it has a tendency to seek out the Earth below us. Water, of course, floats on Earth when it is in its purest form. Pure air belongs above the water, which is where we find it. Fire rises even above air, and so belongs up above.
Empedocles’ four elements can be combined in various ways, and thus, this four element model of matter has remarkable explanatory power. What happens when you set a log on fire? Well, when a log burns, the fire that was inside of it is released. The fire goes up and the log weighs less than before and looks a bit different too (since the fire that was part of it is no longer there, of course).
A boat floats because even though it is made of quite a bit of earth, there is also a lot of air inside. There’s more air than earth overall, meaning that air belonging above water overcomes earth belonging below it. Of course, if the boat springs a leak, it is filled with water rather than air, and the earth can return to its rightful place below the water.
A remarkable number of everyday things can be explained by the idea that matter is composed of only four basic elements, which combine in various ways and have a rightful place in the order of things. It’s wrong, of course. but the success of Empedocles’ model in explaining observations of everyday phenomena serves as an important reminder that in science, models start out as simple as possible until they need to get more complicated.
In today’s world, we know about all sorts of phenomena that Empedocles’ model simply cannot explain. For example, there was simply no way for a four element model to explain electricity. But, of course, Empedocles didn’t know about electricity. Today, our models of matter have become more complicated in order to explain a wider range of known phenomena.
But no matter how complicated models of matter get, they are designed to do one basic thing – explain why different objects and materials have different properties and why they interact as they do.
A golden idea
Imagine playing outdoors with a dog and rolling a ball under the couch in the picture to the dog waiting on the other side. It’s fun for a while, but then you notice that sometimes the ball doesn’t go straight through when you roll it – it gets deflected to the side. Every once in a while, the ball actually comes right back to you.

You’d probably suspect that there was something under the sofa, like extra support legs you can’t see or perhaps some rocks. Even without seeing them, you would know that something is under the sofa. This is the basic idea behind a famous experiment conducted by Ernest Rutherford a little more than a hundred years ago.
Rutherford shot a stream of tiny particles called alpha particles at a thin piece of gold foil. He noticed something remarkable. Most of the alpha particles passed straight through the gold foil, and some of the alpha particles were deflected in different directions.
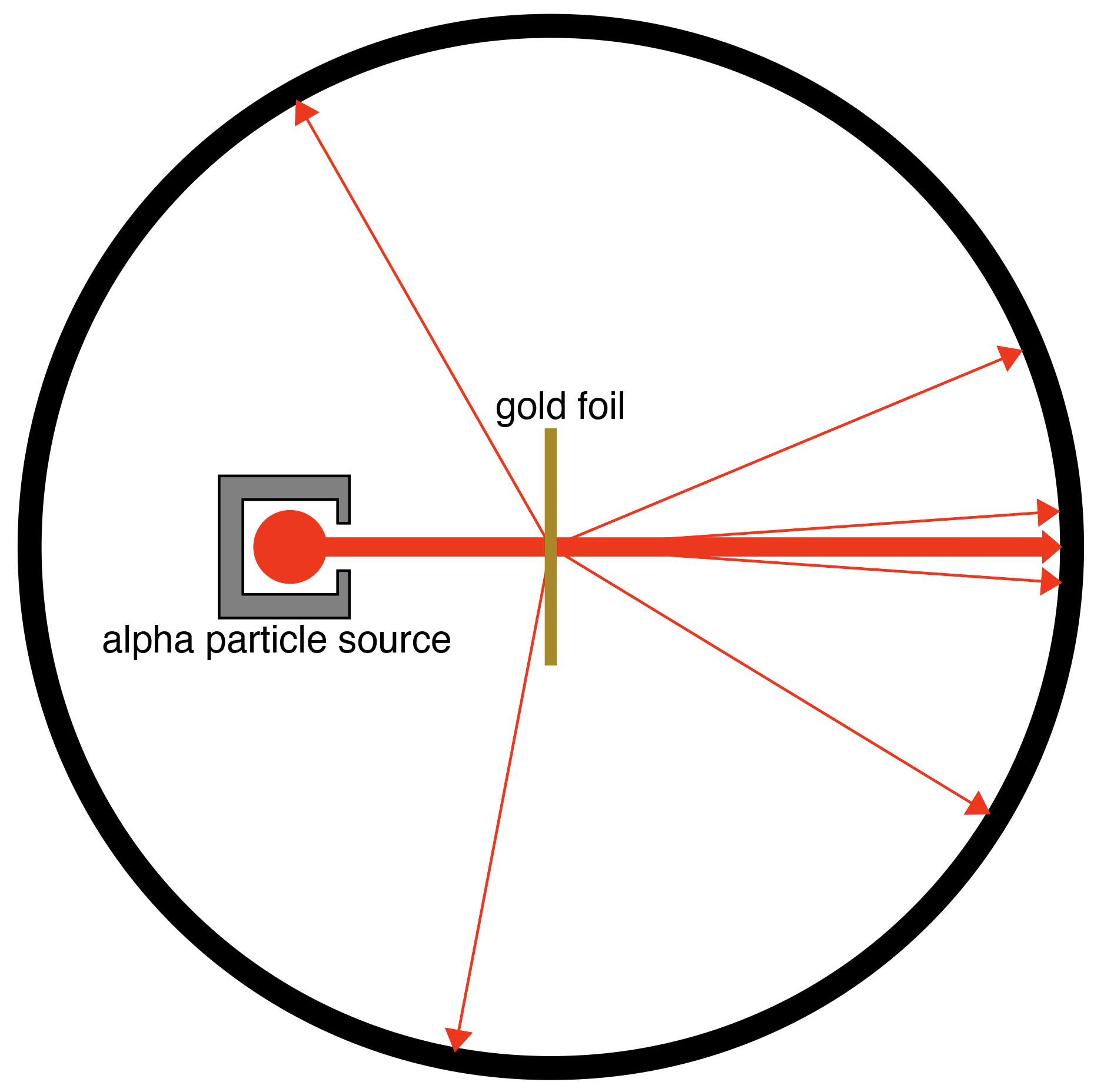
Just like the ball rolling under the couch that was sometimes hitting an object underneath it, Rutherford speculated that most of the foil was actually empty space, and that there was something in the foil that the alpha particles were hitting every once in a while. This something was atoms. Of course, we can look under a sofa and see what the ball is hitting, but atoms are far too small to see. Even though Rutherford didn’t observe atoms directly, he observed very powerful evidence that matter was composed of tiny little particles that he couldn’t see.
Modern imaging techniques have allowed us to much more precisely detect and represent atoms – though we still do not see them directly. This has provided increasingly compelling evidence that everything around us is composed of tiny particles called atoms that interact in various ways.
What is an atom?
Atoms are the basic building blocks of matter. They are constantly in motion, and they attract each other when they are a little distance apart, but repel when they get too close together. All in all, there are about 100 different types of atoms. These are listed on the periodic table of elements.
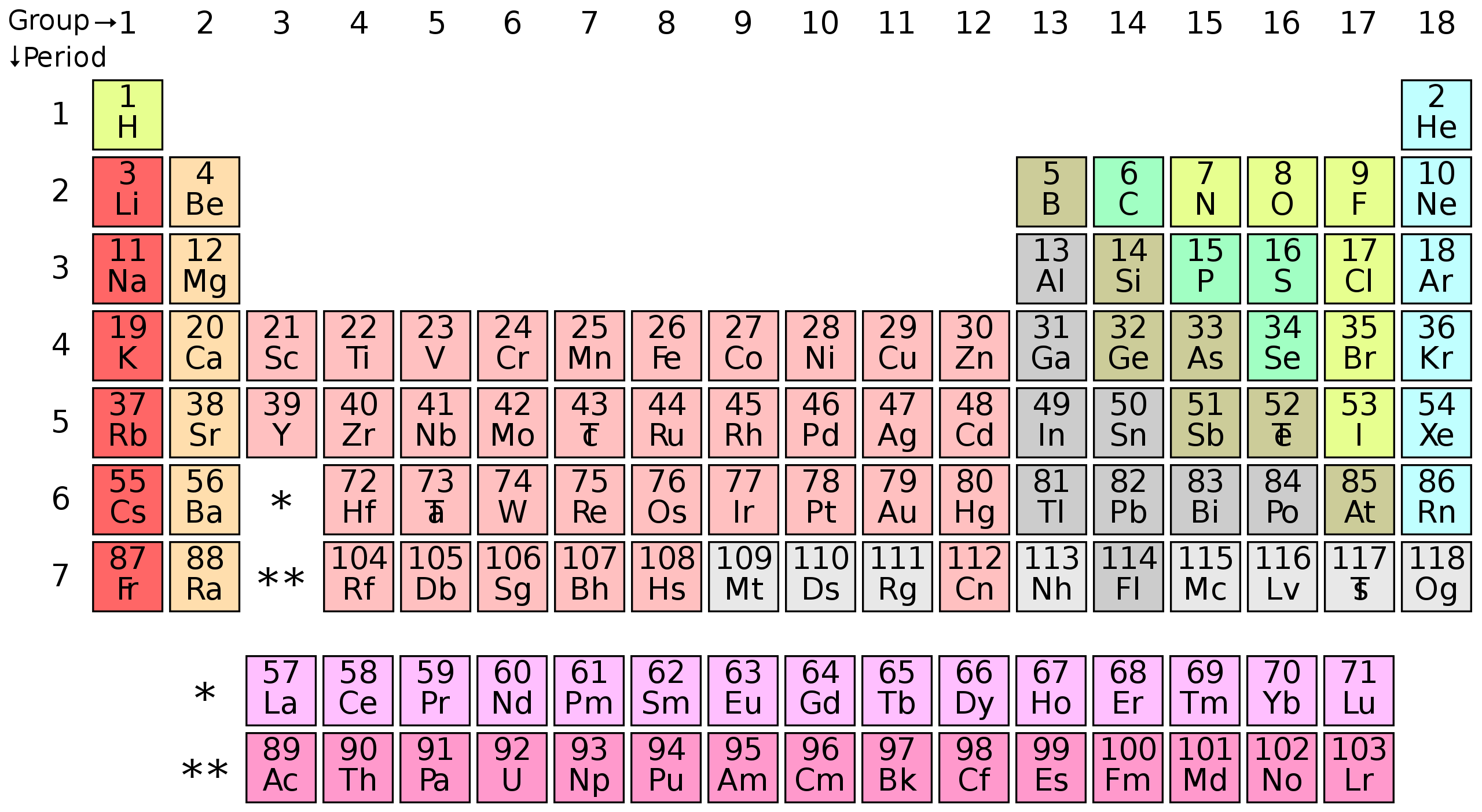
The periodic table is organized as it is because the different types of atoms are similar to each other, and there are patterns in how they interact with other atoms. For example, the atoms in column one are really attracted to atoms in column 17. The atoms in column 18 are hardly attracted to other atoms at all. Everything around us is made of collections of the hundred-or-so types of atoms in the periodic table above.
Atoms in motion
The atoms that make up matter are constantly in motion. We can see evidence for this by doing something as simple as dropping a drop of dye in water.
Of course, we do not see atoms moving as they spread out, or “diffuse”. But if we assume that matter is made of particles that are constantly in motion and constantly colliding with each other, we can construct a simulation that shows how dye diffuses in water. Try the simulation below.
In the simulation, be sure to change the temperature setting.
Important definition
Temperature is a measure of the average kinetic energy of the particles in a substance. Kinetic energy is related to speed. This means that:
As the temperature of a substance increases, the particles that make it up move faster.
Notice that the higher the temperature, the faster the particles move and the faster the dye particles spread out.
Another factor that influences how fast things diffuse is the particle size. If a big particle collides with a small one, the effect on the motion of the small one is much greater, while the effect on the motion of the big one is much less. Use the simulation below to change the number of small (white), medium (green), and large (blue) particles as well as the temperature.
The idea that all matter is made up of particles that are too small to see is one of the most powerful ideas in all of science. This idea is fairly new (about as old as the automobile), and the true explanatory power of this idea is realized when used in conjunction with the practice of modeling to predict and explain phenomena.
Key Takeaways
- All matter is composed of atoms
- Atoms are constantly in motion
- The higher the temperature of a substance, the faster the particles that make it up are moving.
- Atoms are too small to ever be seen, but we can construct models (in which we assume that matter is made of tiny particles that attract if they are close but repel if they are squeezed into each other) that accurately predict and explain phenomena that we observe.
Relevant NGSS Performance Expectations
| 2-PS1-3 | Make observations to construct an evidence-based account of how an object made of a small set of pieces can be disassembled and made into a new object. [Clarification Statement: Examples of pieces could include blocks, building bricks, or other assorted small objects.] |
|---|
| 5-PS1-1 | Develop a model to describe that matter is made of particles too small to be seen. [Clarification Statement: Examples of evidence supporting a model could include adding air to expand a basketball, compressing air in a syringe, dissolving sugar in water, and evaporating salt water.] [Assessment Boundary: Assessment does not include the atomic-scale mechanism of evaporation and condensation or defining the unseen particles.] |
|---|

