1 Overview: Central Nervous System
- Describe the anatomy, structures, and landmarks of the CNS
- Describe malignancies of the CNS
- Describe the simulation process
- Identify commonly used positioning & immobilization devices used for CNS treatments
- Define scan parameters and reference isocenter location for CNS simulations
- Discuss special considerations in CNS patient positioning
- Define treatment borders and how they relate to tumor spread
- Describe tumor volumes and margins of CNS tumors
- Discuss the various treatment procedures of CNS malignancies
- Perform tasks associated with the simulation and treatment of CNS malignancies
Key Terms
Overview: Central Nervous System
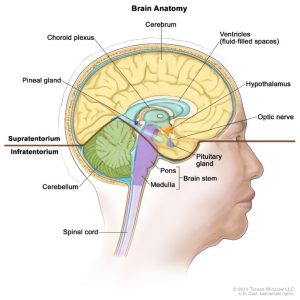
The Central Nervous System (CNS) is comprised of the brain and spinal cord. It is separated from vasculature by the blood-brain barrier (BBB). The CNS receives nutrients that would typically be carried by blood, through the cerebrospinal fluid (CSF). Tumors of the CNS can be categorized as benign or malignant; malignant tumors represent <1% of malignancies in the US. Histologically benign tumors are sometimes treated as malignant due to their location. Nearly 85% of primary CNS tumors will involve the brain, the remainder are found in the spinal cord. In adults, most primary brain tumors are located supratentorial in the cerebrum.
In adults, the most common primary malignant tumors of the CNS are classified as high-grade (anaplastic) astrocytoma’s and glioblastomas (38%). Meningiomas account for 27%; these tumors originate from the 3-layered membrane that protects the CNS. Approximately 90% of these tumors are classified as low-grade or benign. The most common brain tumors are “secondary” or metastatic; 20% to 40% of cancer patients develop brain metastasis in the course of their disease. Metastatic lesions most commonly occur in the cerebral hemispheres, with a single metastasis occurring 40-45% of the time. The most common primary site of disease responsible for brain metastasis is the lung, which occurs in 30-60% of patients; breast cancer and melanoma are also common primary sites. Brain metastases are uncommon in pediatric patients[1].
Primary CNS tumors are the second most common cancer in children, behind leukemia. These tumors are commonly located infratentorial in the posterior fossa. The most common pediatric brain tumor is a low-grade astrocytoma or pilocytic astrocytoma. Radiation is often avoided in children under the age of 3 due to long-term IQ detriments and developmental changes.
The most important prognostic factors include: tumor histology (grade), tumor size and location which influence extent of surgical resection, age – younger patients tend to do better, and Performance Status (PS). No universal staging system is currently in use for CNS tumors, this lack of standardized staging has resulted in confusion. The American Joint Committee on Cancer (AJCC) uses a 3-grade system based on the grade, tumor, metastasis (GTM) classification. The World Health Organization (WHO) has a similar 4-grade system. Lower grade tumors tend to be classified as benign.
You can learn more about this topic by clicking on this link from Learn Oncology.ca.
| Grade | Features of Grade |
| Grade I | Well differentiated, grow slow, classified as benign |
| Grade II | Moderately differentiated, grow slow but may spread to nearby tissues or recur |
| Grade III | Poorly differentiated, grow quickly and spread to nearby tissues – Anaplastic Astrocytoma |
| Grade IV | Undifferentiated, grow and spread very quickly via CSF, necrotic – Glioblastoma Multiform |
Patient Simulation: CNS
Simulation (sim) is preformed after the patient’s consultation and consent process are completed with the physician. The physician issues a sim order that specifies the patient’s treatment site, use of contrast media, immobilization, and scan parameters; these are subject to department protocol and physician preference, but standards exist across the field. Immobilization is especially important in the head and neck area of the body due to the numerous tissue types with varying radio-sensitivities. Radiation therapists should always review the order for accuracy and assess the patient’s ability to achieve the requested position relative to the beam angles required for the treatment. A radiation therapist may need to test several positions and immobilization devices. Achievement of these goals will lead to less patient movement, improved treatment accuracy, and decreased setup time.

Patients are commonly simulated utilizing CT. Patients with brain tumors are typically positioned in the supine position, headfirst, with a thermoplastic mask, knee bolster, feet-banded, and hands clasped or holding a ring. Positioning aides, like the banded feet and ring, help make the patient more comfortable and serve as a reminder to hold still. Thermoplastic masks greatly reduce the errors in the reproducibility of a brain setup; these can be made using a hot water bath or oven. The head rest used should be a comfortable height and keep the patients head in a neutral position – as if they were standing looking straight ahead, matching the curvature of their neck. Markers or “fiducials” are placed laterally on each side of the mask just superior to the ear and midline anteriorly, superior to the orbits.
The radiation therapist is responsible for thoroughly documenting the patients position and set-up instructions with lateral and anterior photographs. Photos should include all immobilization devices and positioning aides. Include closeup images of any fiducials or reference marks, bolus, or complexities in the patient setup.
Special Simulation Considerations: CNS
Patients that are claustrophobic may require premedication, open face masks, and/or the eyes cut out of their mask to allow them to see. Proper coaching, pre-sim education, playing the patients music of choice, or providing them updates throughout the treatment could help keep them calm. Some patients with tumors of the brain may require a safety strap across their arms and chest due to the increased potential of a seizure. If mask-shrinking or facial swelling due to steroid use is a concern, a shim can be placed under the patient’s headrest during mask formation – it must be removed after the mask cools, before the CT. It is important to allow the mask to cool to vendor specifications before removing.
Treatment Volume Localization: CNS
Primary brain tumors commonly spread through local invasion along pre-existing pathways defined by white matter tracts. Most gliomas tend to spread invasively because they do not form a natural capsule. Tumors expand through local invasion; more aggressive gliomas have cells break away into the circulating cerebrospinal fluid (CSF), allowing the tumor to spread to other parts of the CNS. Primary brain tumors do not metastasize through a lymphatic drainage system and rarely metastasize outside of the CNS.
Tumors of the brain are typically treated with lower energy beams, less than 6 MV. Higher energies, have an increased skin-sparing effect – an increased dmax. This increased depth is a disadvantage due to the under dosage caused in the lateral aspects of the brain.
 The treatment margin greatly depends on pathology, the treatment technique selected, and the Organs At Risk (OAR). Even palliative whole brain doses (30 Gy) can result in significant short-term memory deficits and decrease in a patient’s quality of life. Studies have shown that limiting the dose to the hippocampus will greatly improve patient’s side effects and lower the chances of negative neurocognitive declines[2].
The treatment margin greatly depends on pathology, the treatment technique selected, and the Organs At Risk (OAR). Even palliative whole brain doses (30 Gy) can result in significant short-term memory deficits and decrease in a patient’s quality of life. Studies have shown that limiting the dose to the hippocampus will greatly improve patient’s side effects and lower the chances of negative neurocognitive declines[2].
| Organs at Risk (OARs) | TD 5/5 (Whole Organ) | Outcome Associated |
| Lens of Eye | 10 Gy | Cataracts |
| Optic Chiasm | 50 Gy | Blindness |
| Brain Tissue | 47 Gy | Necrosis/infarction |
| Lacrimal Gland | 26 Gy | Dry Eye |
| Optic Nerve | 50 Gy | Blindness |
| Brain Stem | 50 Gy | Necrosis/infarction |
| Spinal Cord | 47 Gy (20 cm) | Myelitis/Necrosis |
| Parotid | 32 Gy | Xerostomia |
| Ear | 30 Gy
55 Gy |
Acute Serous Otitis Chronic Serous Otitis |
Treatment Techniques: CNS
Treatment doses and fraction schemes can vary greatly and depend on the tumor grade, the number of lesions, size and location of the lesion(s), the intent of the treatment (curative vs. palliative), and the patient’s performance status and age. More details are provided in sections: Whole Brain & Primary Brain Tumors, Craniospinal Irradiation (CSI), & Stereotactic Radiosurgery (SRS).
Emerging Technologies & Treatments: CNS
Advances in technology are happening more rapidly than ever before. Today, there are several treatment technologies and devices available that have increased the accuracy and reduced treatment time for CNS malignancies. A few treatment advancements include:
- 6 degrees of freedom tables – Corrections for patient tilt and rotation (Vendor examples: Protura & Hexapod)
- Infrared motion/surface monitoring systems (Vendor examples: AlignRT & Real-time HD Motion Management with Gamma ICON)
Media Attributions
- OER CNS 1 © Terese Winslow LLC is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- brain mask © National Cancer Institute is licensed under a CC BY (Attribution) license
- CNS Brain: Beam Energy Selection © Jared Stiles adapted by Jared Stiles is licensed under a CC BY (Attribution) license
- CNS Brain: Patient Misalignment © Jared Stiles adapted by Jared Stiles is licensed under a CC BY (Attribution) license
- “Adult Central Nervous System Tumors Treatment (PDQ®)–Health Professional Version.” National Cancer Institute, 14 Oct. 2022, www.cancer.gov/types/brain/hp/adult-brain-treatment-pdq. ↵
- Pokhrel, D., Sood, S., McClinton, C., Shen, X., Lominska, C., Saleh, H., Badkul, R., Jiang, H., Mitchell, M., & Wang, F. (2016). Treatment planning strategy for whole-brain radiotherapy with hippocampal sparing and simultaneous integrated boost for multiple brain metastases using intensity-modulated arc therapy. Medical Dosimetry, 41(4), 315–322. https://doi.org/10.1016/j.meddos.2016.08.001. ↵
Breaks in beam output where no monitor units/dose are delivered
a selectively permeable membrane comprised of a phospholipid bi-layer of cells. Drugs that are not lipid soluble may have to be given directly into the spinal cord (intrathecal).
Surgery or cytoreduction to decrease the amount of cancer in the body.
Swelling caused by an excess of fluid in the body
External Auditory Meatus (ear canal)
real-time imaging demonstrating motion and/or function
the process of applying beams, reviewing isodose line, and modifying to avoid critical structures
Delivers stereotactic radiosurgery using precise gamma rays.
multiple treatments per day
fewer fractions at a higher dose
Pressure within the skull (cranium)
the treatment planning process of setting dose constraints on critical structures and the planning system design a treatment plan that meets the plan specifications
Cell death caused by cell injury
at present, no evidence of disease; prevents or protects from metastasis
The first step in the treatment planning process to localize the treatment area. Typically is CT-based and involves the creation of immobilization devices and positioning aides.
fixed; do not change in shape or intensity
Separates the cerebrum from the cerebellum
above the tentorium - cerebrum. Area responsible for speech judgement, thinking and reasoning, problem-solving, emotions and learning.
Includes the area below the tentorium - the cerebellum and brainstem. The cerebellum maintains balance, posture, coordination, fine motor skills, and the brainstem is responsible for autonomic body functions.


