14 Overview: Thorax
Learning Objectives
- Describe the anatomy, structures, and landmarks of the thorax
- Describe malignancies of the thorax
- Describe the simulation process
- Identify commonly used positioning & immobilization devices used for thorax treatments
- Define scan parameters and reference isocenter location for thorax simulations
- Discuss special considerations in thorax patient positioning
- Define treatment borders and how they relate to tumor spread
- Describe the lymphatic drainage and lymph node chains of the thorax
- Describe tumor volumes and margins of thorax tumors
- Discuss the various treatment procedures for thorax malignancies
- Perform tasks associated with the simulation and treatment of thorax malignancies
Key Terms
-
- Akimbo
- Adventitia
- Bellows Belt
- Breathing Ratio
- Dyspnea
- Hemoptysis
- Hila
- Horner's Syndrome
- Mediastinum
- Mesothelioma
- Metaplasia
- Non-Small Cell Lung Cancer (NSCLC)
- Oligometastatic Disease
- Orthopnea
- Pancoast Tumor
- Pneumonitis
- Prophylactic Cranial Irradiation (PCI)
- Respiratory Gating
- Skip Metastasis
- Small Cell Lung Cancer (SCLC)
- Superior Vena Cava Syndrome (SVCS)
- Stereotactic Body Radiation Therapy (SBRT)
- Topogram
- Virtual Isocenter
The thorax contains the heart, major blood vessels, and lungs; the ribs, breastbone, and spine support and protect the organs. The esophagus is also included in this unit, although it is considered part of the digestive system. The right lung is divided into three lobes, an upper, middle, and lower. The left lung is divided into two lobes, an upper and lower. The apex, or top of the lung, is located a few centimeters above the clavicle, and the base, or bottom of the lung, sits at the diaphragm. When an individual inhales, the diaphragm moves inferiorly, allowing the lungs to fill with air. When an individual exhales, the diaphragm moves superiorly, pushing air out. The heart is located left of midline within the mediastinum.
The trachea (windpipe) extends from the larynx to the bronchi; it carries air to and from the lungs during respiration. At the inferior portion of the trachea, the bronchi bifurcate, or divide, into primary bronchi; this location is called the carina. The carina is around the fifth or sixth thoracic vertebra, but can be anywhere from T4-T7, depending on the patient’s anatomy and body habitus. The carina serves as an important landmark in radiation therapy that can be seen in 2D and 3D imaging.
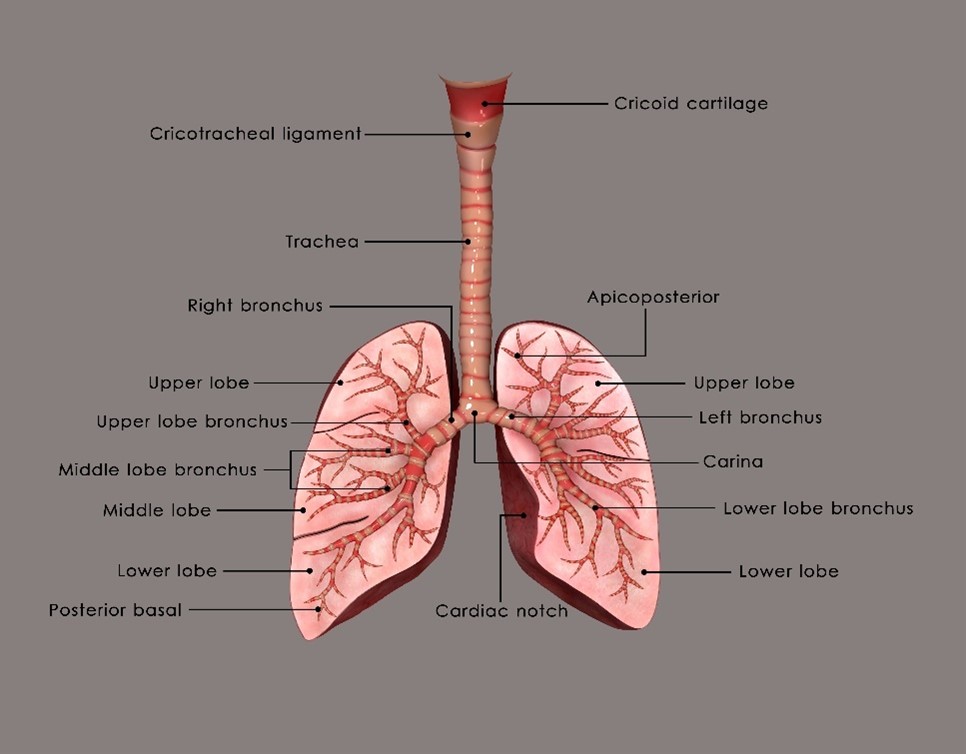
The lungs are separated midline by the mediastinum, composed of the heart, thymus, trachea, great vessels, esophagus, and lymph nodes. The mediastinum can be divided into the superior and inferior compartments. The hila of the lungs contain blood vessels, bronchi, and lymphatics. The superior vena cava (SVC) is also important in radiation therapy; it is a vital venous channel for the return of blood to the heart from the upper thorax, head, neck, and upper extremities. The SVC is surrounded by structures in the anterior mediastinum and numerous lymph nodes.
Lung and bronchus cancer is the second most common cancer diagnosed in men and women and is the leading cause of cancer death in the United States. The leading etiologic factor is smoking, while radon exposure is the leading environmental risk factor, second overall. The average age of diagnosis of lung cancer is 65 years of age.
Lung cancers can arise anywhere in the lungs. One example is a Pancoast tumor, which is a tumor that occurs at the apex or the superior aspect of the lung. This cancer was named after Henry Pancoast in 1932 and is also called a superior pulmonary sulcus tumor. Pancoast tumors are rare (they only account for 3-5% of lung cancers) and may present with unique symptoms because of their location that may involve one-sided shoulder pain or Horner's syndrome. Horner’s syndrome includes the same side drooping of the eyelid (ipsilateral ptosis), lack of facial sweating (anhydrosis), and pupil constriction (miosis).
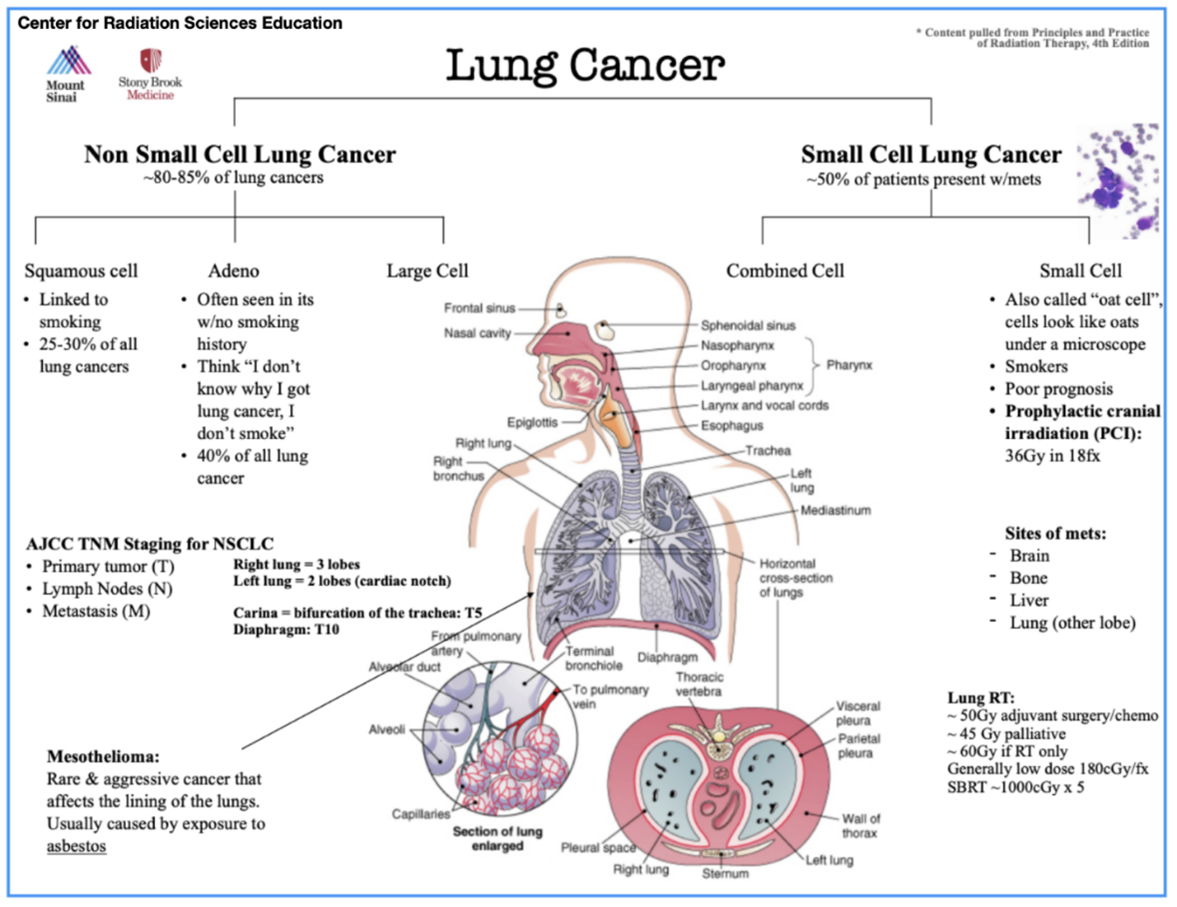
Lung cancer is treated with curative or palliative intent and is divided into two main categories: Non-small cell lung cancer (NSCLC) and Small cell lung cancer (SCLC).
NSCLC accounts for roughly 80-85% of all lung cancers; it includes the histology’s of squamous cell carcinomas, adenocarcinomas, and large cell tumors. Squamous cell carcinomas are more common in patients with a long smoking history. Tobacco damages cilia enabling chemical carcinogens to irritate the lung’s tissues and cause damage. In contrast, adenocarcinoma is the most common lung cancer in patients with no smoking history. These tumors tend to arise in the outer parts of the lung. Large cell tumors are the least common and tend to grow and spread quickly. NSCLC is treated with surgery when the tumor is localized and in its early stages. Higher dose radiation (>60 Gy) and concurrent chemotherapy should be offered for optimal control in more advanced stages or when the tumor is inoperable.
SCLC, also called “oat cell,” is a rapidly spreading pathology with a high probability of metastasis at diagnosis (50%). Due to its rapid spread, multiagent chemotherapy and radiation therapy offer the best chance for a complete response. Most SCLCs are located in the central or hilar regions of the lungs. Prophylactic cranial irradiation (PCI) is often recommended as a preventative measure for brain metastases. More information on prophylactic radiation therapy can be found in the CNS section.
Mesothelioma is a rare and aggressive cancer that affects the lining of the lungs. The mesothelial cells secrete a serous fluid to lubricate between the chest wall and the lungs. Cancers here are often associated with asbestos exposure.
Surgery, if a treatment option, provides the best chance to cure lung cancers. Radiation and chemotherapy are commonly used due to the advanced stage at diagnosis and aggressiveness of these malignancies. Cisplatin is the chemotherapy of choice when treating lung cancers.
Patients with cancers of the thorax are positioned supine with arms above their head in a wing board, arm board, or vacloc, and a knee bolster. The patient is uncovered above the waist, removing any jewelry. They are centered and straightened on the table, and reference isocenter marks are placed anteriorly and laterally on stable points, not too close to the armpit or skin folds. Reproducible and precise immobilization is essential in treating lung lesions.
Often, intravenous (IV) contrast is requested for scans of the thorax. Radiation therapists should follow departmental contrast protocols concerning patient education and pre-contrast assessment. Patients will initially follow the machine’s breathing instructions for the topogram; this allows the therapist to choose the proper scanning parameters. Generally, scans of the thorax extend from the mid-neck to the inferior costal margin or the third lumbar vertebra.
Simulations of the thorax can present with numerous challenges. The most common consideration for cancers of the thorax is tumor motion caused by respiration. Several treatment technologies and devices are available to reduce tumor motion; patient selection for each is important. Some devices utilize abdominal compression that restricts the depth of inspiration for the patient. Other techniques coach the patient how to breathe and include a breath-hold technique, active breathing control (described in the breast unit), and respiratory gating using a 4D CT.
Department protocols will vary based on the devices and technology available, but commonly 4D scans are taken to evaluate target motion. A 4D scan uses “time” as the fourth dimension; they capture multiple images of the patient’s anatomy at different phases of the breathing cycle, creating a dataset representing the range of motion. Patients with tumor motion greater than 5 mm will benefit from motion management treatment, like gating or compression. Both techniques will spare normal tissues by reducing the treatment field size.
During the simulation, patients are educated to establish their eligibility for compression or gating. If a 4D scan is an option, breathing instructions are explained to the patient. It is important to note the patient’s nominal breaths per minute and breathing ratio. These measurements determine the patient’s breathing pattern during the simulation. Respirations are monitored throughout the scan via a belt or tracking device.
Departmental protocols will vary. The University of Iowa acquires an expiration breath-hold scan with contrast for their primary dataset and a 4D scan. The 4D scan is performed using the patient’s established breaths per minute and breathing ratio. After the scan, a physicist or dosimetrist evaluates the CT slices for any artifact. Another option is to obtain a max inspiration and an expiration scan to determine the total amount of motion possible. A free-breathing scan is used for patients that are not good candidates for compression or gating. These scans help determine target volumes and assess tumor motion for treatment planning.
Through analysis of the 4D CT dataset, the radiation oncology team can identify the specific phases of the breathing cycle when the tumor is most stable and accurately target those phases for gated treatment. Most treatments deliver the radiation at the bottom of the patient’s breathing cycle (exhalation). For example, when the beam is on, the gating window may be set at 40% expiration to 40% inspiration. Gated treatments require a system that tracks the patient’s breathing cycle while connected to the treatment delivery system. These treatments take longer because the beam stops when the tumor is outside the treatment volume, relying on the patient to breathe consistently. Established workflows should exist in each facility.
Other challenges for treatments of the thorax are patient position and physical ability. For example, if a patient cannot raise their arms above their head, planning beams to safely reach their tumor may be difficult. Therapists should inform the physician and dosimetry; the best arm position may be akimbo. The akimbo position will move the arms away from the thorax, reducing the chance they will receive dose and open up the thorax to more beam angle options. Patients with COPD or large thorax tumors may have difficulty breathing laying flat; elevating the patient’s head with an incline board or vacloc may be necessary.
Treatment Volume Localization: Thorax
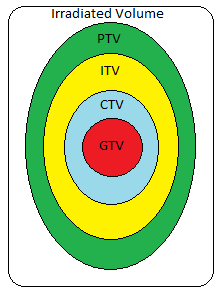
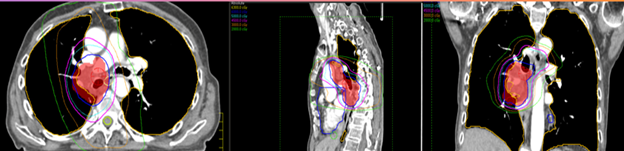
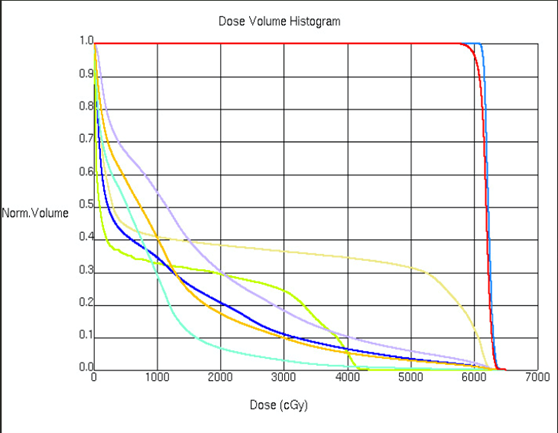
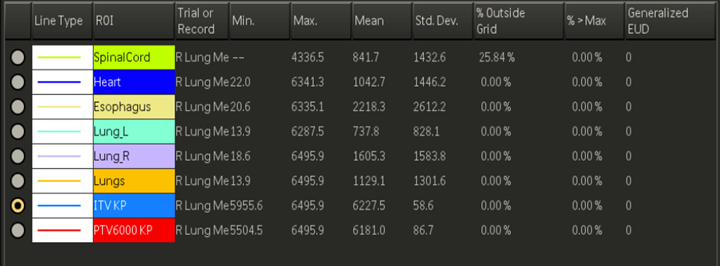
Treatment design for thorax malignancies is based on the primary tumor’s size, location, proximity to critical structures, and lymphatic drainage. Commonly affected nodes include the hilar and mediastinal nodes, but lymphatic drainage depends on the tumor’s location. Patients that are not candidates for respiratory monitoring with a free-breathing CT simulation, will have about a 2 cm expansion from the Internal Target Volume (ITV). The ITV encompasses the CTV with a margin for motion. Patients that are 4D candidates, the PTV is an expansion of 5-8 mm from the ITV.
|
OAR
|
TD 5/5 (Whole Organ)
|
Outcome Associated
|
|
Spinal Cord
|
45 or 47 Gy
|
Myelitis/Necrosis
|
|
Lung
|
18 Gy
|
|
|
Heart
|
40 Gy
|
Pericarditis
|
|
Esophagus
|
55 Gy
|
Stricture/Perforation
|
|
Brachial Plexus
|
55-60 Gy
|
Nerve Damage
|
Treatment Techniques Overview: Lung
During the verification simulation appointment, first, position the patient to their 3-point reference (CT) isocenter. Indicated shifts from dosimetry are then applied to move the patient to the planned treatment isocenter. Depending on department preference, new set-up marks may be placed on the patient to indicate the planned isocenter and to eliminate daily shifts. Otherwise, shifts from the reference 3-point marks are applied daily. New marks should only replace existing marks when they can be placed on stable surface anatomy. SSD’s should be documented and table parameters recorded. Patients with lateral isocenters (>8 cm off midline) may require a lateral off-set to reduce clearance issues. For example, if a patient has a right lateral lung lesion greater than 8 cm off midline, placing the patient closer to the left edge allows the right side of the body to be closer to the center of the treatment table. A virtual isocenter may be needed for conebeam (CBCT) clearance if daily imaging is indicated. For safety, always verify gantry clearance before exiting the room.
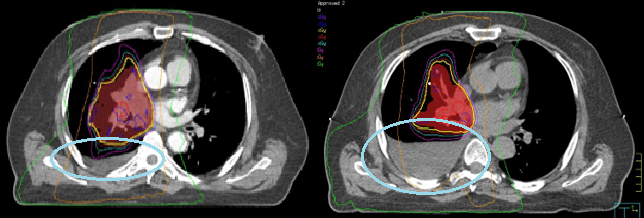
Most treatments today utilize some form of daily Image Guided Radiation Therapy (IGRT). If the tumor is well defined, align to the planning target volume (PTV) and check bone and carina for verification. The PTV includes margins for uncertainties in beam alignment, tumor motion, and patient positioning. The thoracic spine is the most stable boney anatomy for alignment, especially for posteriorly located lesions. Ensure the spinal cord contour is within the vertebral foramen and verify the carina. For mediastinal tumors, align the carina first and then check the spine.
- Palliation (AP/PA): beam arrangement was historically used for lung treatments but is now commonly reserved for palliation. The initial AP/PA fields were treated to a dose of 45 Gy. If doses above 45 Gy are required, the beam arrangement is modified to obliques termed an “off-cord boost” to avoid overdosing the spinal cord and subsequent transverse myelitis.
- 3D Conformal Radiation Therapy (3DCRT): The size of the PTV and its location to structures dictate the beam arrangement. A three-field conformal plan is used for early-stage disease (Stages 1 & 2). The three fields are an anterior oblique, a posterior oblique, and a lateral. Wedges are used on the oblique beams to compensate for the curvature of the chest wall. Multi-leaf collimator (MLC) shielding conforms each beam shape to the PTV. More beam angles are added with increasing disease stage to improve the overall dose to the tumor and reduce the dose to the normal lung volume and surrounding tissues.
- Volumetric Modulated Arc Therapy (VMAT)/IMRT: Most treatments of the thorax use daily conebeam CT (CBCT) imaging and IMRT or VMAT treatment. These highly conformal plans reduce the dose to normal tissues and maximize the dose to the PTV. Plans are designed by setting dose limits and parameters in the planning system and the computer optimizes a treatment plan to the specifications.
|
Pre-op
|
45-50 Gy in 1.8-2 Gy/fx*
|
|
Post-op
|
50-54 Gy in 1.8-2 Gy/fx*
|
|
Definitive/Curative
|
Treat nodes to 45 Gy then boost ipsilateral lymph nodes to 60-70 Gy*
|
|
Palliative
|
20 Gy in 5 fx or 30 Gy in 10 fxs
|
|
Hyper fractionation (Limited stage SCLC)
|
1.5 Gy BID to 45 Gy over three weeks
|
|
Stereotactic Body Radiation Therapy (SBRT)
|
25 Gy in 5 fx with a minimum of 40 hours between fractions OR
50 Gy in 5 fx with a minimum of 40 hours between fractions* |
|
Emergent – Superior Vena Cava Syndrome/SVCS
|
Initial fx doses of 3-4 Gy/fx for approximately 2-4 days until improvement in symptoms THEN fractions of 1.8-2.0 Gy/fx to a total dose of 45-60 Gy*
|
Emerging Technologies & Treatments: Thorax
Surface monitoring systems assist during patient setup and monitor the patient’s position throughout treatment. Surface monitoring systems can track surface motion and position with <1 mm accuracy. These systems use a 3D model of the patient acquired during their CT simulation. A red light projects onto the patient’s skin surface and records their surface position. The systems monitor the pitch, yaw, and roll of the patient. Pitch is the back elevation, yaw is the hip adjustment, and roll is the patient’s rotation.
During treatment setup, surface monitoring systems assist in reproducing the patient’s position. After daily imaging and completing table adjustments, a new image capture is taken for treatment. The image is used as a baseline to monitor patient movement during treatment – this is required daily. Some clinics utilize surface monitoring systems that automatically interrupt the radiation beam if the patient moves out of tolerance.
Motion monitoring systems like respiratory gating (previously described) and live imaging continue to make advances.
Proton Therapy: Motion improvement techniques for pencil beam scanned proton therapy should be evaluated and selected based on organ motion. Organ movements to be assessed for proton therapy include those of the target and all organs in the potential proton beam path. For example, treating the lower esophagus using posterior fields where the diaphragm moves severely impacts the delivered beam range relative to the treatment target.
Media Attributions
- Lung anatomy © 7activestudio is licensed under a All Rights Reserved license
- Lung Cancer ppt slide © Mount Sianai Center for Radiation Sciences Education at Stony Brook University is licensed under a All Rights Reserved license
- Girl patient lies on computed tomography bed and scanning lungs for diagnose lung cancer in medical clinic. CT x-ray examination of lung cancer in medical © Rabizo Anatolii is licensed under a All Rights Reserved license
- Planning target volumes © Jared Stiles is licensed under a CC BY (Attribution) license
- Right lung isodose distribution change with increasing fluid © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- IMRT lung © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- IMRT lung DVH © Jared Stiles is licensed under a CC BY (Attribution) license
- IMRT lung DVH 2 © University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
Arms bent with hands on the hips
The outermost layer of connective tissue surrounding an organ or vessel
A belt worn on the patient that monitors their breathing as they inhale and exhale
The breathing ratio refers to the relationship between the time spent exhaling and inhaling during the breathing cycle. It is calculated by dividing the time spent exhaling by the time spent inhaling. For example, if a patient spends twice as much time exhaling as inhaling during the breathing cycle, the breathing ratio would be 2:1.
difficulty breathing
coughing up blood
The area where the blood and lymph vessels, bronchi, and nerves enter and exit the lungs
A rare disorder that presents with a drooping of the upper eyelid, constricted pupil, and absence of sweating on the face. An apical lung tumor can cause it.
The space between the lungs that contains the heart, thymus, trachea, esophagus, lymph nodes, and great vessels.
An aggressive form of cancer affecting the lining of the lungs, usually caused by exposure to asbestos.
When one type of mature tissue is replaced by another type of mature tissue not typically found there
The most common type of lung cancer comprised of three main types: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma
refers to the spread of cancer cells from the primary site to only one or two other parts of the body, resulting in new tumors
Shortness of breath when lying down flat and improves when sitting or standing up
Cancers that start in the apex of the lung
Inflammation of the lungs
Used for patients diagnosed with small cell lung cancer to prevent the disease from spreading to the brain
A breathing technique used during radiation treatment to target the tumor when it isn't moving
A common metastatic pattern in NSCLC occurring when lateral lymph nodes have disease without involvement in the central lymph nodes
An less common but more aggressive form of lung cancer that spreads quickly
When the superior vena cava is compressed and partially or completely blocked, it is usually caused by cancer
A radiation technique that precisely targets the tumor and delivers high doses of radiation to the cancer, usually given in five or fewer treatments
2-D x-ray image taken with a CT scanner (aka CT scout view)
A designated point near the target necessary to avoid collisions between the patient and machine.

