28 Overview: Leukemia
Learning Objectives
- Describe the leukemia and the goals associated with treatment
- Describe the simulation process
- Identify commonly used positioning & immobilization devices used for TBI treatments
- Discuss special considerations in TBI patient positioning and planning
- Describe the treatment setup process and treatment considerations
- Describe treatment doses and fractionation schema used for TBI
- Perform tasks associated with the simulation and treatment of CNS malignancies
Key Terms
Overview: Leukemia
Leukemias are cancers that originate from the early blood-forming stem cells of the bone marrow – typically the white blood cells. These cells do not function normally and rapidly proliferate blast cells. The blast cells crowd the bone marrow and reduce the number of normal, functioning blood cells leading to symptoms. Common symptoms include fatigue, frequent infections, anemia, easy bruising, bleeding, and enlarged lymph nodes or spleen. Normal bone marrow consists of <5% blast cells; acute leukemia is present if >20% the bone marrow is blast cells.
Acute leukemias are fast-growing while chronic leukemias are slow-growing; most childhood leukemias are acute. These cancers are further classified by cell-type as myeloid or lymphoid; both are types of white blood cells. Myeloid cells play a critical role in immunity and tissue repair and act as first-responders to pathogens and cellular injury. Types of myeloid cells include neutrophils, monocytes, eosinophils, and basophils. Lymphoid cells produce antibodies responsible for a faster immune response from previously encountered pathogens. Cell types include T-lymphocytes, that mature in the thymus, and B-lymphocytes, that mature in the bone marrow. B-cell leukemia is more common (85%).
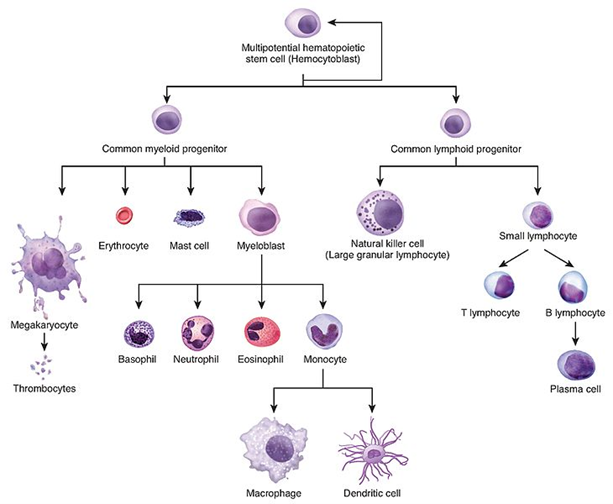
Subtypes include:
| ALL | Acute Lymphoblastic Leukemia | Most common pediatric cancer (75%); peak incidence ages 2-5; Accounts for 1 in 3 pediatric cancers |
| CLL | Chronic Lymphocytic Leukemia | Typically of B-cell origin; Most common adult leukemia; Is not linked to radiation exposure; no cure, but can be managed for some time |
| AML | Acute Myeloid Leukemia | Most common acute subtype in adults and about 1/3 of leukemias overall |
| CML | Chronic Myeloid Leukemia | Can enter an acute phase or blast crisis; Characterized by the presence of the gene abnormality – the Philadelphia chromosome. |
Less common subtypes include: Hairy Cell Leukemia, Prolymphocytic Leukemia, Adult T-Cell Leukemia/Lymphoma, Large Granular Lymphocytic Leukemia, Chronic Neutrophilic Leukemia, T-Cell Prolymphocytic Leukemia, B-Cell Prolymphocytic Leukemia, Biphenotypic Leukemia, T-Cell Large Granular Lymphocytic Leukemia, Acute Promyelocytic Leukemia, Acute monocytic Leukemia, Acute Erythroid Leukemia.
Treatment for leukemia depends on the type and stage of the disease. Since leukemia is a systemic disease, systemic therapy is most effective. Chemotherapy and immunotherapy have significantly improved survival and are the standard of care. Radiation therapy is considered for some leukemias, usually of acute subtype, with high-risk features. It is also sometimes used in the treatment of neuroblastomas, Ewing’s sarcoma, lymphomas, and other hematological and autoimmune disorders.
Total body irradiation (TBI) achieves the immunosuppression needed to reach nadir in preparation for a bone marrow transplant. Transplant types include allogenic, autologous, and syngeneic. Due to these patients being immunocompromised, they are in reverse isolation.
Additional treatment sites include the cranial cavity and scrotum for male ALL patients. The scrotum is a known sanctuary site and is typically treated with a testicular boost via clinical electron setup in one fraction and 2-4 Gy during the TBI course. Cranial irradiation is also sometimes employed for CNS leukemias, high risk, or patients in relapse. Treatment doses are 12-18 Gy at 180-200 cGy/fraction to the whole brain.
Patient Simulation: Leukemia
There are many different technologies and setups to accomplish a whole-body treatment. When positioning patients for total body irradiation, it is important to consider the treatment technology available and the department’s protocol. A common conventional treatment setup and technique utilizes a linac at an extended treatment distance; the simulation is performed clinically in the treatment room. Other techniques are described in the Emerging Technologies & Treatment section.
For a conventional setup, begin by positioning the patient on the treatment table in a decubitus position in an upper and lower vaclok or one large vaclok. The arm on the patient’s downside is positioned up with their hand at the top of their head. The torso is perpendicular to the table with their arm at the side. Bend their knees and hips slightly to provide stability. A cushion or rolled towels under the patient’s head and between their knees will improve comfort. Raise the treatment table and transfer the patient to the TBI treatment table.
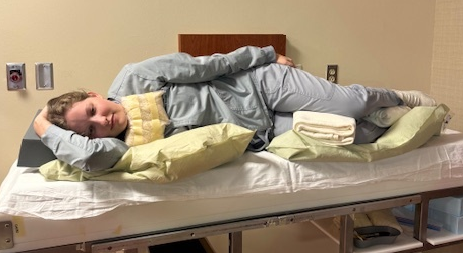
Once the patient is moved to the TBI treatment table, move them into position at the calibrated treatment distance. Rotate the gantry, directing the light-field towards the treatment wall and patient. Open the jaws to 40×40 cm and rotate the collimator to 45 degrees to take advantage of the increased length of the diagonal aspect of the field. The central ray should fall near the umbilicus; ensure the patient fits inside the radiation field with adequate light-field falloff at the head and feet. Use a coronal laser to align the patient such that approximately half their body is anterior and posterior to the laser – mark the patient.

Special Simulation Considerations: Leukemia
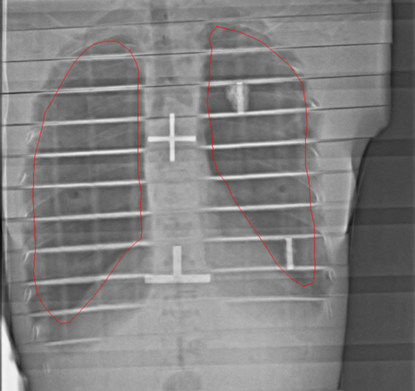
The light field might not accurately represent the radiation field at the extended distance. It is important physics has identified the radiation field size and marked it on the wall. Verify the patient shadow is within the radiation field at their head and feet.
Fractionated treatments typically require the use of partial transmission lung blocks to reduce the dose to the lungs by 50%. The lungs are extremely radiosensitive, a lower dose will help reduce the risk of interstitial pneumonitis which can be fatal. The mounting system for the blocks can vary by department; a plexiglass tray with slots to adjust the location of the blocks is a common system. The tray also acts as a beam spoiler and should cover the central axis (CAX) of the beam. Ensure correct lung block placement by placing an imaging cassette or film behind the patient’s thorax. Image the patient using an appropriate number of monitor units (~20-50 MU depending on patient size, distance, and department protocol).
The video above demonstrates the patient’s position, the equipment’s position, plexiglass tray and lung blocks, and an imaging system for block localization.
Treatment Volume Localization: Leukemia
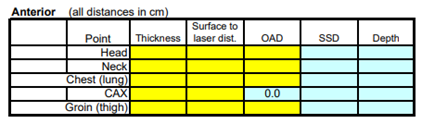
For a conventional setup, dose calculation is completed by measuring the patient’s thickness at their head, neck, chest, abdomen (at the central axis), and thighs. Measurements should include the depth from the anterior and posterior surfaces to the coronal laser. Patients simulated using CT, volumes are accounted for in the treatment planning system.
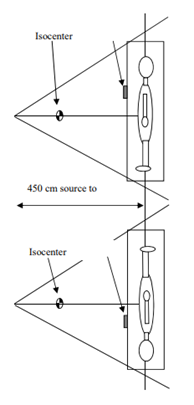
At an extended treatment distance, it is necessary to add a beam compensator to maintain beam flatness and symmetry. Additional compensators to shape the beam are commonly necessary to account for variations in patient thickness and inhomogeneities in tissue density. For example, the patient’s thinner body parts like the head, neck, and lower extremities may require compensation to receive a uniform dose. Compensators are attached to the flattening compensator previously described. Additionally, patients can be treated supine with lateral beams and their arms at their sides to act as a compensator and reduce the dose to the lungs.
Thermoluminescent dosimeters (TLDs) or MOSFETs can be used to monitor patient dose and make ensure dose uniformity. Side effects of treatment include nausea vomiting, diarrhea, decreased secretions, erythema, alopecia. Organs at risk during TBI include the lungs, lens of the eyes, gonads, kidneys, liver, bowel, and brain. Late effects in children could include endocrine deficiencies, stunted growth, and IQ deficits.

Treatment Techniques: Leukemia
Most fractionation schemes for acute leukemias are twice-daily 2 Gy fractions, given 6 hours apart, over 3 days to a total dose of 12 Gy using a 6 MV beam. Patients who cannot tolerate standard fractionation due to advanced age, other comorbidities, or relapse are offered reduced-intensity TBI and receive 2-4 Gy in 1-2 fractions. A low-dose rate of 5-10 cGy per minute and pre-medication will help reduce side acute side effects. During the first fraction, dose measurements should be taken using TLD’s or an ion chamber at each point patient measurements were taken on posterior and anterior surfaces.
Emerging Technologies & Treatments: Leukemia
VMAT TBI can deliver a more uniform dose to the entire body targeting bone marrow and lymphatics and spare structures like the lungs, heart, brain, and kidneys. Patients are scanned supine headfirst, supine feetfirst, and whole-body in a head and shoulder mask with a lower vaclok or a whole body vaclok. The treatment is delivered using multiple beams and isocenters with feathered match lines.
TomoTherapy TBI treats the patient’s whole body one slice at a time. This is great to achieve a uniform dose to the whole body and reduce dose to critical structures like the lungs and kidneys.
- Total Marrow Irradiation (TMI) can be used to target the patient’s bone marrow. This allows for dose escalation (12-20 Gy) which could improve outcomes without increasing toxicities.
- Total Marrow Lymphoid Irradiation (TMLI) incorporates the major lymphatics into the treatment volumes (included in TMI video above).
Media Attributions
- Hematopoiesis © OpenStax, Wikimedia Commons is licensed under a CC BY (Attribution) license
- TBI patient position © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- TBI light field © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- Lung blocks © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- TBI measurement chart © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- AP/PA TBI beams with lung blocks © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- TBI flattening filter with lead compensators © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
replaces a patient's damaged or diseased bone marrow with healthy stem cells from a donor
involves collecting, storing, and then returning a patient's own healthy stem cells to them after treatment
used to increase dose in superficial tissues and improve dose homogeneity
immature blood cells that develop from stem cells in the bone marrow
A medical procedure in which damaged or destroyed bone marrow is replaced with healthy stem cells that can develop into new blood cells.
BMT is often used to treat diseases such as leukemia, lymphoma, multiple myeloma, and certain blood or immune disorders.
The imaginary line that runs through the center of the radiation beam.
blending the dose daily between fields to avoid overdose of a critical structure like the spinal cord
suppression of the body's immune system and its ability to fight infections
Metal-Oxide-Semiconductor Field-Effect Transistors are used as dosimeters for measuring radiation dose. MOSFET dosimeters are highly sensitive, small, and capable of providing real-time dose measurements
lowest point; usually in reference to a patients blood counts after each cycle of chemotherapy or other treatment
return or regrowth of cancer after a period of improvement or remission
also known as protective isolation, is a method to protect immunocompromised patients from exposure to infections
an area in the body where cancer cells can evade systemic treatments
precursor cells, blood stem cells originate in the bone marrow
transplanting healthy stem cells from an identical twin into a patient to replace damaged or diseased bone marrow
An additional dose of radiation (typically 2–4 Gy) delivered to the scrotum and testes in male patients with acute lymphoblastic leukemia (ALL) to reduce the risk of testicular relapse. The testes can act as a sanctuary site for leukemic cells.
Total Body Irradiation - delivering a therapeutic dose of radiation to the entire body done for some leukemia patients in preparation for a bone marrow transplant.
devices used to measure ionizing radiation exposure by capturing and storing energy when exposed to radiation
a procedure that replaces a patient's unhealthy bone marrow with healthy stem cells
center of the treatment beam

