30 Sarcomas: Overview
Learning Objectives
- Describe the anatomy of bone and soft tissues
- Describe malignancies of bone and soft tissues
- Describe the simulation process for sarcomas of various locations
- Identify commonly used positioning & immobilization devices used based on treatment location
- Define scan parameters and reference isocenter location
- Discuss special considerations in patient positioning
- Describe treatment borders and how they relate to tumor spread
- Describe tumor volumes and treatment margins
- Discuss the various treatment procedures an technologies available in the treatment of sarcomas
- Perform tasks associated with the simulation and treatment of CNS malignancies
Key Terms
Overview: Sarcomas
Sarcomas are cancers of bones, muscles, and other connective tissues that originate from mesenchymal tissues. They are rare and comprise less than 1% of all cancers. While the direct cause is unknown, genetic factors play a significant role in pediatric diagnoses while environmental factors like radiation and chemical exposures increase the risk later in life.
Sarcomas tend to spread initially by local invasion in a longitudinal direction. Lymph node spread is not common; instead, metastasis most often occurs hematogenously. Common metastatic sites include the lungs, bones, brain, and liver.
Sarcomas represent a therapeutic challenge as they are generally radioresistant and chemo resistant – however there are exceptions like Ewing sarcoma. Surgery is typically the preferred treatment option, but resection may be difficult or not possible due to a tumor’s location and proximity to critical structures. Treatment options include:
- Surgery: Surgery may not be an option if there is invasion of nerves, blood vessels, or fractures. Limb-sparing surgery is preferred for extremities to maintain quality of life; it produces similar survival as amputation.
- Chemotherapy: Is commonly used for osteosarcoma, Ewing sarcoma, and advanced disease – most beneficial for younger patients.
- Radiation Therapy: May be used for benign tumors and tumors that cannot be surgically removed, offers chance at improved local control.
The table below summarizes the main differences between carcinomas and sarcomas:
| Feature | Carcinoma | Sarcoma |
| Frequency | Much more common (~90% of all cancers) | Rare (~1% of all cancers) |
| Common Sites | Skin, lungs, breast, colon, prostate, pancreas, liver, etc. | Bones, muscles, tendons, cartilage, fat, blood vessels, connective tissue |
| Examples | Adenocarcinoma, squamous cell carcinoma, basal cell carcinoma | Osteosarcoma, chondrosarcoma, leiomyosarcoma, liposarcoma |
| Cell Type | Arises from epithelial cells | Arises from mesenchymal (connective tissue) cells |
| Histologic Appearance | Cells arranged in nests or sheets; may form glands | Spindle-shaped or pleomorphic cells; resembles connective tissue |
| Growth Pattern | Often forms solid tumors with distinct borders | May grow as deep-seated masses, often less well-defined |
| Typical Spread | Primarily through lymphatic system | Primarily through the bloodstream (hematogenous spread) |
| Common Age Group | More common in older adults | Can occur at any age, but often younger individuals |
| Response to Therapy | Typically, responsive to chemotherapy and radiation | Often less responsive; surgery is primary treatment |
Sarcomas are commonly divided into two groups:
- Bone sarcomas (.2% of cancers) and
- Soft tissue sarcomas (.7% of cancers)
Bone sarcomas
Bone sarcomas are primary malignant tumors originating from skeletal tissues like bone and cartilage. Most bone cancers are metastatic or secondary in nature. Common sites that metastasis to the bones are cancers of the lungs, breasts, and prostate. Metastatic prostate lesions can be osteoblastic while most other sites tend to be osteolytic lesions.
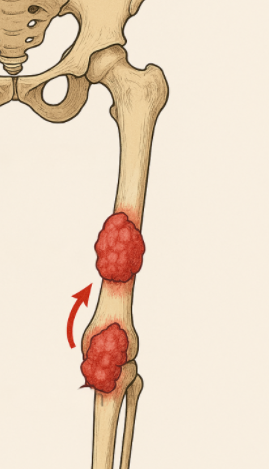
Bone sarcomas are more common in children, adolescents, and young adults. Presenting symptoms are location dependent, but commonly include mass, pain, swelling, fracture, compressed nerves, and weight loss. Skip metastasis are separate tumors found within the same bone (more common) or across a joint space; they’re rare and typically found with osteosarcomas. The presence of skip metastasis (mets) carries a worse prognosis.
Sarcomas are staged using the AJCC’s (American Joint Committee on Cancer) TNM (Tumor, Node, Metastasis) system. In addition, bone sarcomas are also graded using a 3-grade system based on the level of differentiation. Another commonly used system is the Enneking staging system developed by the Musculoskeletal Tumor Society (MSTS), which considers the tumor’s grade, extent, and metastatic status.
| Parameter | Category | Description |
| Grade (G) | Low (G1) / High (G2) | Reflects tumor aggressiveness |
| Tumor Extent (T) | Intracompartmental (T1) / Extracompartmental (T2) | Whether the tumor is confined to or has extended beyond the anatomical compartment of origin |
| Metastasis (M) | M0 / M1 | Absence or presence of distant metastasis |
The most common types of primary bone cancer include:
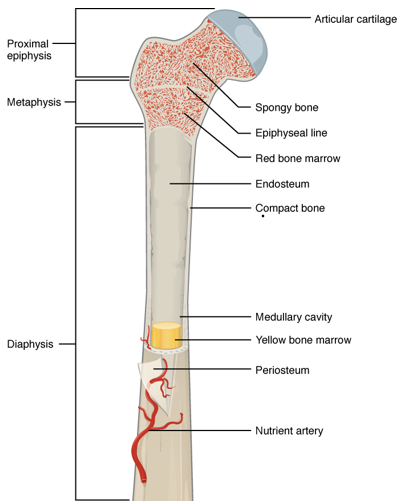
- Osteosarcoma
- Most common bone cancer overall and in children/young adults.
- Often arises in the metaphysis of long bones (e.g., femur, tibia).
- High-grade; often treated with surgery and chemotherapy, but RT (Radiation Therapy) is used for inoperable or margin-positive cases.
- Chondrosarcoma
- Cartilage-forming tumor found in the metaphysis of long bones.
- Most common bone sarcoma in adults, typically age 50+.
- Radioresistant – surgery is mainstay; RT only used for inoperable cases or high-grade subtypes.
- Ewing Sarcoma
- Affects children and young adults; can occur in bone or soft tissue.
- Often found in the diaphysis of long bones, the pelvis, and ribs.
- Highly responsive to chemoradiation; RT is commonly part of definitive or adjuvant treatment.
Soft tissue sarcomas (STS)
Soft tissue sarcomas (STS) are tumors that develop in the extra-skeletal connective tissue such as fat, muscle, nerve, fibrous tissue, joints, blood or lymphatic vessels, or deep skin tissues. The most common location for soft tissue sarcomas is in the extremities, but they can be found to a lesser extent in the trunk, retroperitoneum, and head and neck. Patients with STS typically present with a painless mass; it may be accompanied by joint pain, phlebitis, and weight loss.
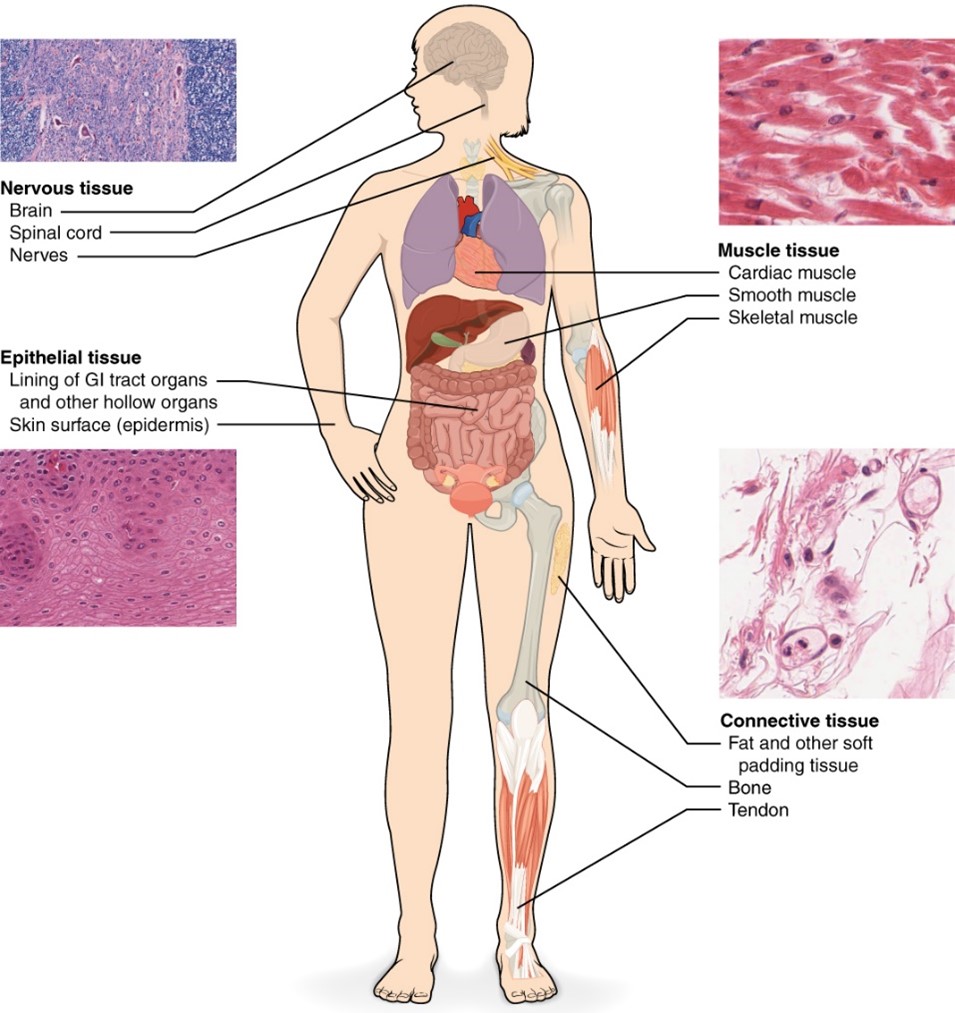
The AJCC’s TNM staging system is used and has established different systems based upon the tumors location – either retroperitoneal or trunk and extremities. Additionally, STSs are assigned a grade using the French scoring system which considers the tumors differentiation (1-3), mitotic count (1-3), and necrosis (0-2).
The most common types of STS include:
- Rhabdomyosarcoma
- Most common STS in children (age 5-20) and overall.
- Originates in skeletal muscle (voluntary).
- Undifferentiated pleomorphic sarcoma (UPS; formerly malignant fibrous histiocytoma – MFH)
- Most common adult STS – age 50-70.
- Fibrosarcoma
- Arises from fibrous tissue in adults aged 20-50 years old.
- Liposarcoma
- Arises from fat tissue, common in thigh or retroperitoneum.
- Common in adults 40-60 years old.
- Leiomyosarcoma
- Originates in smooth muscle (involuntary) along the GI tract and vascular system.
- Synovial sarcoma
- Affects young adults, often near joints,
- Can spread lymphatically.
- Kaposi sarcoma
- Can occur in immunocompromised patients typically who are over 50
- Appears as purple lesions.
Patient Simulation: Sarcomas
CT simulation for sarcoma treatment planning is often performed with intravenous (IV) contrast to enhance tumor delineation when clinically appropriate. MRI can provide superior soft tissue detail and is recommended to aid in target definition. Ideally, it should be performed in the treatment position and fused with the planning CT whenever feasible. A PET/CT (Positron Emission Tomography/Computed Tomography) may also offer additional information on tumor extent and should be considered. Scan parameters are generous and will be determined by the radiation oncologist. Generally, 2mm slices are used, but in locations near critical structures, 1mm slices may be preferred.
The optimal treatment position depends on the tumor location and should prioritize patient comfort, reproducibility, and adequate clearance for both imaging and treatment delivery. Beam arrangements and target coverage should be carefully considered before finalizing the patient’s position and fabricating any immobilization devices.
- Thorax or Abdomen:
- Use a wing board or other immobilization device to position the arms above the head.
- Head and Neck:
- Use a headrest with a thermoplastic mask for immobilization.
- Upper Extremities:
- A vaclok should immobilize the affected extremity either above the head or in an akimbo position (away from the body). Turn the patient’s head away from the affected arm/shoulder.
- Lower Extremities and Pelvis:
- A vaclok is typically used to secure the limbs or pelvic region. Feet immobilization is important when treating the pelvis to keep the hips rotated the same each day. When treating male pelvis and thigh sarcomas, fashioning a sling under the patient before they lay down with a pillowcase can help pull the genitals away from the treatment field.
- Prone Positioning:
- Considered for posterior or superficial lesions when feasible. Ensure patient comfort and reproducibility, accounting for physical limitations.
Special Simulation Considerations: Sarcomas
- Bolus application: Scars and/or drain sites are often included in the treatment volume and may require bolus. Position the patient so that the bolus can either be applied after setup or placed within the VacLok prior to molding.
- Beam access: Position the affected extremity away from other body parts to maximize available beam angles. Avoid excessive displacement that could create clearance or collision issues.
- VacLok formation: Mold the VacLok snugly around the body part to ensure support and reproducibility. Do not obstruct lasers or optical surface monitoring systems (e.g., VisionRT). Before marking or scanning, remove the extremity from the mold, place a pillowcase or sheet inside the mold, and then reposition the extremity. This improves reproducibility and makes daily positioning easier.
- Isocenter location: Ensure that all relevant anatomy and immobilization devices are included within the field of view (FOV) for treatment planning, density accuracy, and machine clearance.
- On the treatment machine, verify both imaging and treatment clearance before finalizing setup.
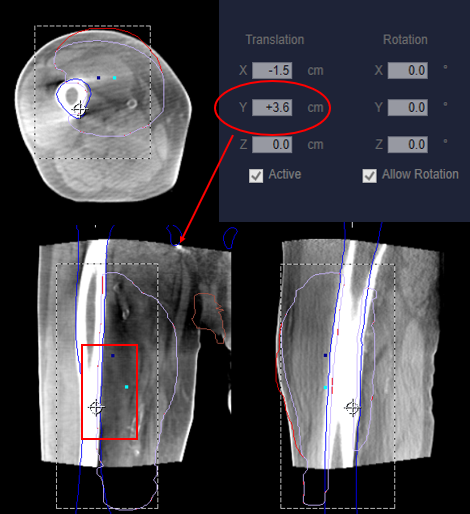
- Lateral offsets: Laterally located isocenters may require table offsets laterally to achieve imaging clearance. Note: Adjusting patient position laterally to achieve imaging clearance will likely reduce the visible treatment area on CBCT due to its smaller FOV.
- Longitudinal offsets: If the treatment area is centered within a long field, for example the femur, a longitudinal shift may be helpful to visualize anatomy and confirm correct positioning in the superior/inferior directions – see Figure 4.
Reproducing the patient’s simulation position for treatment can be especially challenging. If OSMS (Optical Surface Monitoring System) positioning is unavailable and a 3-point setup cannot be achieved due to the opposite extremity obstructing one of the lateral lasers, use a 2-point setup.
To accurately reproduce a 2-point setup:
- Request a DRR (Digitally Reconstructed Radiograph) or SSD at gantry zero from dosimetry before the verification simulation.
- Align and level the patient using the 2-points, the anterior and lateral lasers on the effected side.
- Once aligned, check the SSD. Set the SSD if it is off.
- If off, reposition the patient’s level by rolling the extremity and realigning the two points until matched. *Try not to adjust your table vertical*
- Align and check the SSD. Repeat until marks align and SSD matches what was planned.
After imaging, if the extremity rotation is incorrect, reposition. Once correct alignment is established, set the couch vertical for future treatments. Position the patient by rotating the extremity to match the lateral level, then straighten using the anterior laser for final positioning; verify SSD.
Treatment Volume Localization: Sarcomas
Sarcomas tend to grow locally early on spreading to surrounding tissues. Eventually, they gain access to the vascular system and can metastasize hematogenously throughout the body, most commonly to the lungs; lymphatics are not commonly involved. Radiation can be preoperative (neoadjuvant) or postoperative (adjuvant). Local control and survival outcomes are generally comparable.
| Surgery type | RT Dose | Pros | Cons |
| Preoperative Radiation Therapy | 45–50.4 Gy | Better tissue oxygenation = increased sensitivity; can reduce tumor size and make the surgery less invasive | Can cause wound healing issues after surgery; total size and extent of the disease is unknown before RT |
| Postoperative Radiation Therapy | 60–66 Gy (especially if margins are close/positive or if unresectable) | Extent of disease is known, may result in smaller treatment fields or more targeted RT | Requires higher doses due to hypoxia, increased potential late effects, delayed wound healing |
*Doses may need to be lower due to organs at risk near the treatment volume. For example, sarcomas of the abdomen may be limited to closer to 50 Gy.
When treating bone sarcomas, margins are generous to incorporate potential microscopic spread and are typically 1.5-2 cm beyond the GTV (Gross Tumor Volume). For STS, the margins may be larger (~4 cm longitudinally) or include the entire muscle compartment with a 2 cm margin. Patients have an increased risk of skin toxicity, pathologic fractures, and soft tissue fibrosis.
| Organ / Structure | Endpoint (Complication) | TD 5/5 (Gy) |
| Bone | Necrosis / Fracture | 60 Gy (whole organ) |
| Joint | Stiffness / Necrosis | 50 Gy (whole organ) |
| Muscle / Soft Tissue | Fibrosis / Contracture | 60 Gy (whole organ) |
| Skin | Chronic dermatitis / Atrophy | 50 Gy (whole organ) |
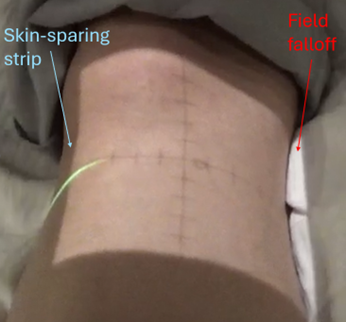
When treating extremities, it is critical to maintain an unirradiated strip of tissue to avoid circumferential fibrosis and maintain lymphatic drainage. Therapists can verify this by reviewing the isodose lines on the CBCT for IMRT plans or checking the light field on the patient for 3D-CRT (Three-Dimensional Conformal Radiation Therapy) plans. Other considerations include avoiding growth plates in pediatric patients and reducing unnecessary dose to joints.
Treatment Techniques: Sarcomas
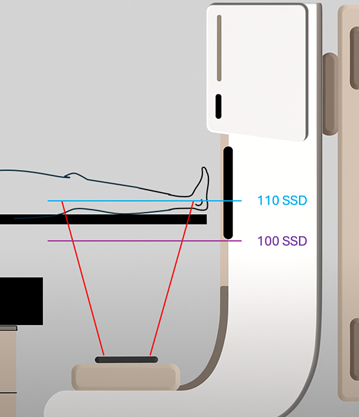
3D-CRT plans can provide adequate coverage and limit dose to critical structures. Most modern treatments utilize IMRT (Intensity-Modulated Radiation Therapy) or VMAT (Volumetric Modulated Arc Therapy) techniques. Sometimes, the treatment volume may exceed the maximum field size – this necessitates an extended distance treatment or field-matching. Field-matching is achieved by using the MLCs to “feather” or blend the dose between adjacent fields or by using IMRT techniques.
Emerging Technologies & Treatments: Sarcomas
Proton Therapy: Allows for highly conformal dose delivery, taking advantage of the Bragg peak to reduce radiation exposure to surrounding healthy tissues. This is particularly beneficial for tumors near critical structures and is often preferred for pediatric patients to increase normal tissue sparing and minimize long-term side effects.
MR-LINAC (Magnetic Resonance Linear Accelerator): MRI-guided linear accelerators provide superior soft tissue visualization compared to conventional imaging. This enables improved tumor localization and assists in precise daily treatment alignment, enhancing the accuracy of dose delivery.
Adaptive Radiation Therapy (ART): ART allows for daily modification of the treatment plan based on changes in tumor size, position, or surrounding anatomy. This approach helps maintain optimal target coverage while sparing nearby critical structures.
Surface-Guided Radiation Therapy (SGRT): Using systems such as OSMS or VisionRT, SGRT (Surface-Guided Radiation Therapy) tracks the patient’s surface in real time for setup and intra-treatment monitoring. This improves positioning accuracy, reduces reliance on skin marks, and helps detect motion during treatment.
Media Attributions
- Figure 1; skip mets sarcoma © Jared Stiles
- Figure 2; bone anatomy adapted by Jared Stiles is licensed under a CC BY (Attribution) license
- Figure 3 Soft Tissues is licensed under a CC BY (Attribution) license
- Figure 4; long. offset © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- Sarcoma video cover © University of Iowa Radiation Therapy program
- Figure 5 skin sparing field © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- Figure 6. IMRT skin sparing © The University of Iowa Radiation Therapy Program is licensed under a CC BY (Attribution) license
- Figure 7 extended distance © Jared Stiles is licensed under a CC BY (Attribution) license
Treatment given after the initial treatment of cancer to remove remaining cancer cells or to prevent recurrence
A type of cancer that begins in epithelial cells, which line the surface of organs, glands, and body structures (such as skin, lungs, breast, and digestive tract).
The shaft or central part of a long bone, composed mainly of compact bone and bone marrow.
A staging system for musculoskeletal tumors (particularly bone and soft tissue sarcomas) that helps guide treatment planning and predict prognosis.
The end portion of a long bone, which articulates with adjacent bones and contains the growth plate in children.
Used for soft tissue sarcomas, based on differentiation, mitotic count, and necrosis.
Describes the degree of differentiation of tumor cells compared to normal cells, indicating how aggressive a tumor is likely to be. Higher grade = more abnormal and faster growing.
A surgical approach that removes a bone tumor while preserving the limb, reconstructing the area with bone grafts, prosthetics, or other materials instead of amputation.
The successful prevention of tumor growth or recurrence at the original disease site after treatment (e.g., surgery, radiation).
The region between the diaphysis and epiphysis, often the site of active bone growth and where many primary bone tumors arise.
These are primitive, multipotent stem cells that can develop into a variety of connective tissue cell types
A group of muscles, nerves, and blood vessels surrounded by a tough connective tissue sheath (fascia) that separates it from other groups. Compartmental anatomy is important in tumor resection and limb-sparing surgery.
The treatment given before the main cancer treatment
Refers to forming new bone or osteoblasts and are commonly seen in prostate cancer
Refers to bone lesions that cause bone destruction or resorption, leading to weakened or radiolucent areas on imaging. Often seen in lung, breast, and kidney metastases.
vein inflammation, often associated with pain and redness along the affected vein
Refers to the period before surgery or treatments given in preparation for surgery.
Refers to the period before surgery or treatments given in preparation for surgery.
Refers to tumors that do not respond well to radiation
A common metastatic pattern in NSCLC occurring when lateral lymph nodes have disease without involvement in the central lymph nodes
A type of cancer that arises from mesenchymal (connective tissue) cells, which form the body’s bones, muscles, fat, cartilage, blood vessels, and fibrous tissues
Cells that develop into connective tissues, including bone, cartilage, and fat.
A common cancer type arising from epithelial cells, affecting organs like the skin, lungs, and breast.
A rare cancer originating from mesenchymal (connective) tissues such as bone, muscle, fat, and blood vessels.
separate tumors found within the same bone or across a joint space; typically found with osteosarcomas
A staging system for bone sarcomas considering grade, extent, and metastasis.